The Future of Vaccines Adjuvants understanding modern vaccines












- Slides: 52

The Future of Vaccines Adjuvants (understanding modern vaccines) Leonard Friedland, MD Vice President, Scientific Affairs and Public Health GSK Vaccines 12 th Statewide Immunization Ohio Conference November 16, 2016

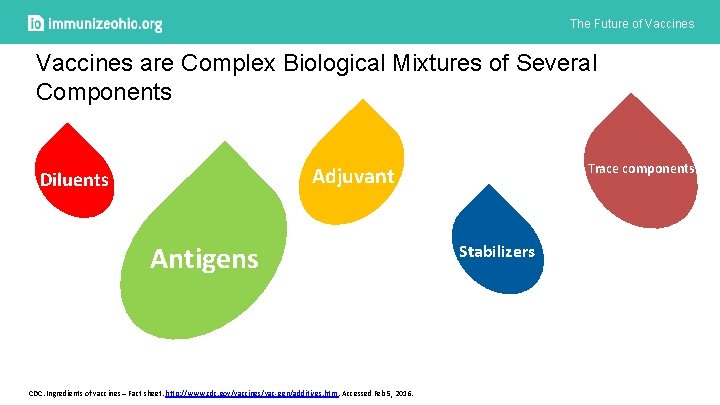
The Future of Vaccines are Complex Biological Mixtures of Several Components Trace components Adjuvant Diluents Antigens CDC. Ingredients of vaccines – Fact sheet. http: //www. cdc. gov/vaccines/vac-gen/additives. htm. Accessed Feb 5, 2016. Stabilizers

The Future of Vaccines Adjuvant • From Latin, adiuvare: to aid • Pharmacological/immunological agent that modifies the effect of other agents • Substances that enhance or shape the immune response • Immunological adjuvants added to vaccines stimulate the host immune system’s response to target antigen, but do not themselves confer immunity • Old technology, made new Garçon N, et al. Understanding modern vaccines, Perspectives in vaccinology, Vol 1, Amsterdam: Elsevier; 2011; Chapter 4: p 89 -113.

The Future of Vaccines 1920 s Major discoveries: adjuvants Discovery of adjuvants by Gaston Ramon Copyright, Wellcome Library, London Use of aluminium salts as vaccine adjuvants by Alexander Glenny Copyright, Wellcome Library, London Bonanni et al. Understanding modern vaccines, Perspectives in vaccinology, Vol 1, Amsterdam: Elsevier; 2011; Chapter 1: p 1– 24

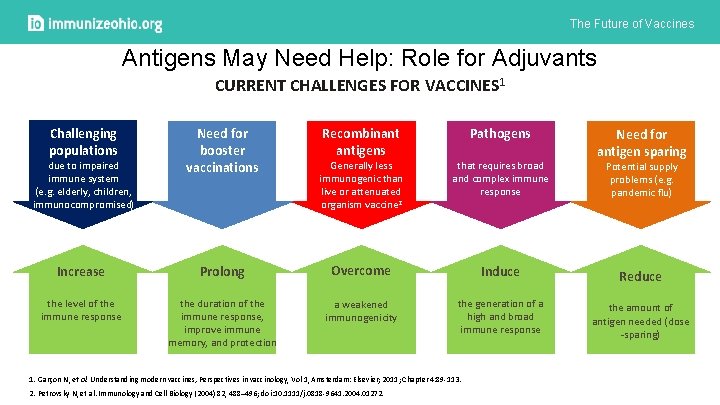
The Future of Vaccines Antigens May Need Help: Role for Adjuvants CURRENT CHALLENGES FOR VACCINES 1 Challenging populations due to impaired immune system (e. g. elderly, children, immunocompromised) Need for booster vaccinations Recombinant antigens Pathogens Generally less immunogenic than live or attenuated organism vaccine 2 that requires broad and complex immune response Increase Prolong Overcome Induce the level of the immune response the duration of the immune response, improve immune memory, and protection a weakened immunogenicity the generation of a high and broad immune response 1. Garçon N, et al. Understanding modern vaccines, Perspectives in vaccinology, Vol 1, Amsterdam: Elsevier; 2011; Chapter 4: 89 -113. 2. Petrovsky N, et al. Immunology and Cell Biology (2004) 82, 488– 496; doi: 10. 1111/j. 0818 -9641. 2004. 01272. Need for antigen sparing Potential supply problems (e. g. pandemic flu) Reduce the amount of antigen needed (dose -sparing)

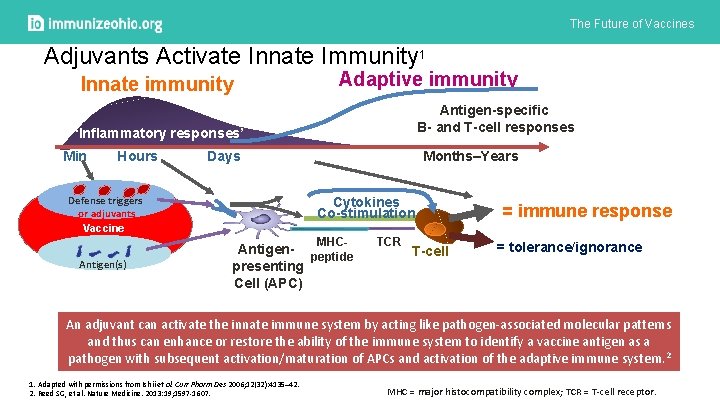
The Future of Vaccines Adjuvants Activate Innate Immunity 1 Innate immunity Adaptive immunity Antigen-specific B- and T-cell responses ‘Inflammatory responses’ Min Hours Days Defense triggers or adjuvants Vaccine Months–Years Cytokines Co-stimulation MHC- Antigen(s) Antigen- peptide presenting Cell (APC) TCR T-cell = immune response = tolerance/ignorance An adjuvant can activate the innate immune system by acting like pathogen-associated molecular patterns and thus can enhance or restore the ability of the immune system to identify a vaccine antigen as a pathogen with subsequent activation/maturation of APCs and activation of the adaptive immune system. 2 1. Adapted with permissions from Ishii et al. Curr Pharm Des 2006; 12(32): 4135– 42. 2. Reed SG, et al. Nature Medicine. 2013: 19; 1597 -1607. MHC = major histocompatibility complex; TCR = T-cell receptor.

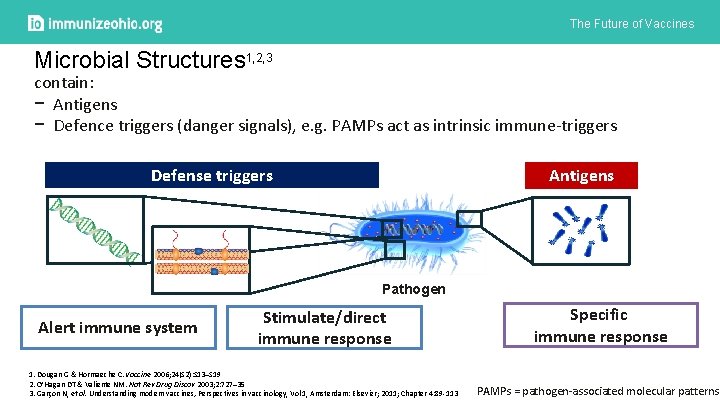
The Future of Vaccines r Microbial Structures 1, 2, 3 contain: − Antigens − Defence triggers (danger signals), e. g. PAMPs act as intrinsic immune-triggers Defense triggers Antigens Pathogen Alert immune system Stimulate/direct immune response 1. Dougan G & Hormaeche C. Vaccine 2006; 24(S 2): S 13–S 19 2. O’Hagan DT & Valiente NM. Nat Rev Drug Discov 2003; 2: 727– 35 3. Garçon N, et al. Understanding modern vaccines, Perspectives in vaccinology, Vol 1, Amsterdam: Elsevier; 2011; Chapter 4: 89 -113 Specific immune response PAMPs = pathogen-associated molecular patterns

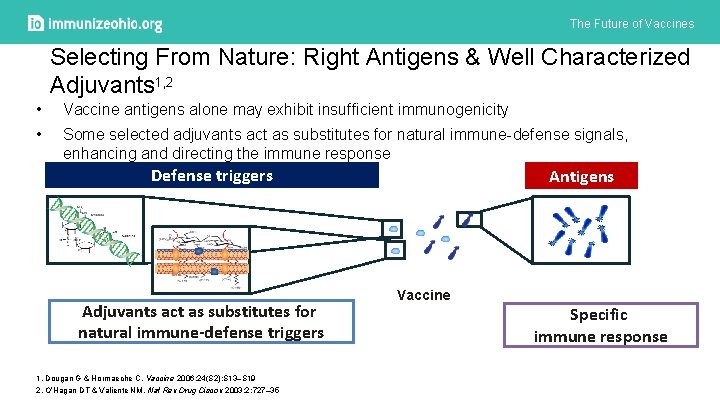
The Future of Vaccines Selecting From Nature: Right Antigens & Well Characterized Adjuvants 1, 2 • Vaccine antigens alone may exhibit insufficient immunogenicity • Some selected adjuvants act as substitutes for natural immune-defense signals, enhancing and directing the immune response Defense triggers Adjuvants act as substitutes for natural immune-defense triggers 1. Dougan G & Hormaeche C. Vaccine 2006; 24(S 2): S 13–S 19 2. O’Hagan DT & Valiente NM. Nat Rev Drug Discov 2003; 2: 727– 35 Antigens Vaccine Specific immune response

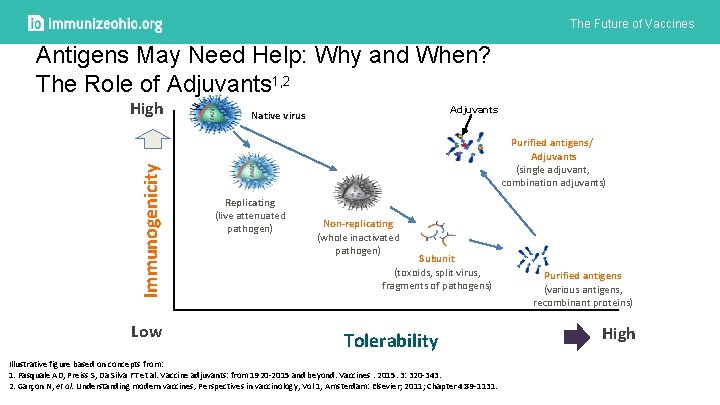
The Future of Vaccines Antigens May Need Help: Why and When? The Role of Adjuvants 1, 2 High 6 Adjuvants Native virus Immunogenicity 5 Purified antigens/ Adjuvants (single adjuvant, combination adjuvants) 4 3 Replicating (live attenuated pathogen) 2 Non-replicating (whole inactivated pathogen) Subunit (toxoids, split virus, fragments of pathogens) 1 0 Low Category 1 Category 2 Category 3 Tolerability Illustrative figure based on concepts from: 1. Pasquale AD, Preiss S, Da Silva FT et al. Vaccine adjuvants: from 1920 -2015 and beyond. Vaccines. 2015. 3: 320 -343. 2. Garçon N, et al. Understanding modern vaccines, Perspectives in vaccinology, Vol 1, Amsterdam: Elsevier; 2011; Chapter 4: 89 -1131. Purified antigens (various antigens, recombinant proteins) Category 4 High

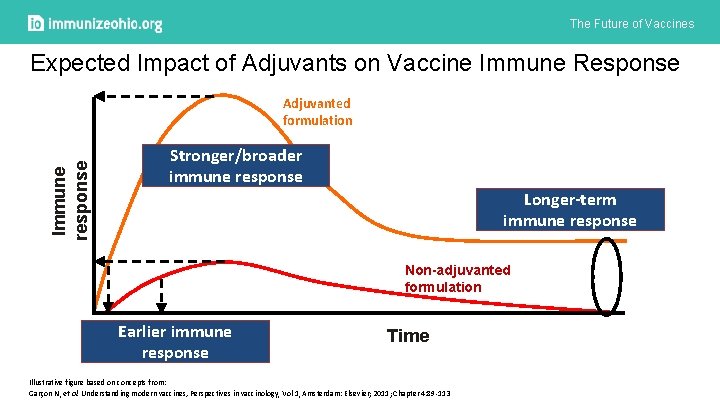
The Future of Vaccines Expected Impact of Adjuvants on Vaccine Immune Response Immune response Adjuvanted formulation Stronger/broader immune response Longer-term immune response Non-adjuvanted formulation Earlier immune response Time Illustrative figure based on concepts from: Garçon N, et al. Understanding modern vaccines, Perspectives in vaccinology, Vol 1, Amsterdam: Elsevier; 2011; Chapter 4: 89 -113

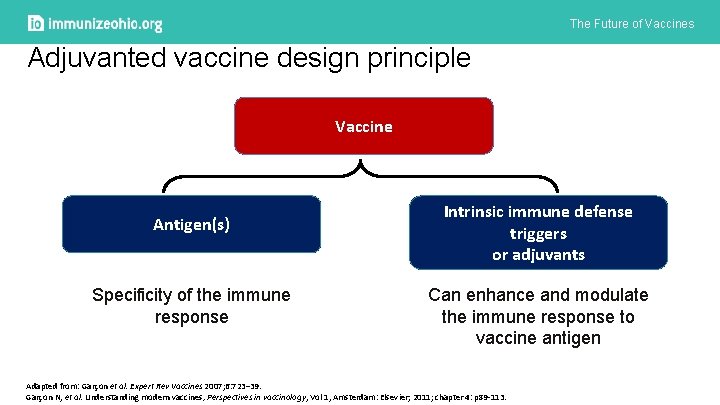
The Future of Vaccines Adjuvanted vaccine design principle Vaccine Antigen(s) Specificity of the immune response Intrinsic immune defense triggers or adjuvants Can enhance and modulate the immune response to vaccine antigen Adapted from: Garçon et al. Expert Rev Vaccines 2007; 6: 723– 39. Garçon N, et al. Understanding modern vaccines, Perspectives in vaccinology, Vol 1, Amsterdam: Elsevier; 2011; chapter 4: p 89 -113.

The Future of Vaccines General Adjuvant Mode of Action 12

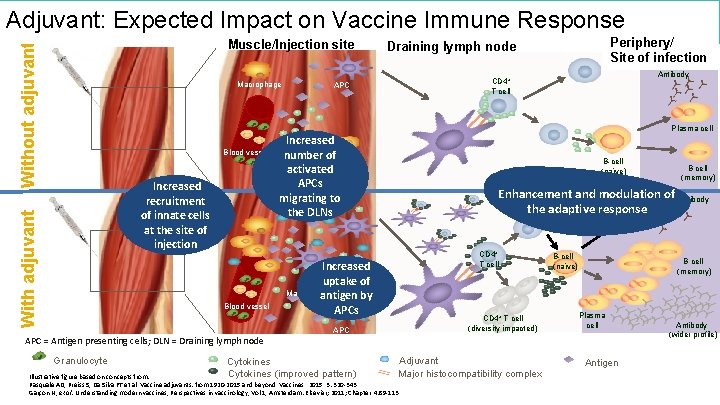
The Future of Vaccines Adjuvant: Expected Impact on Vaccine Immune Response Without adjuvant Muscle/Injection site Macrophage Periphery/ Site of infection Draining lymph node Antibody CD 4+ T cell APC Plasma cell Blood vessel With adjuvant Increased recruitment of innate cells at the site of injection Blood vessel APC = Antigen presenting cells; DLN = Draining lymph node Granulocyte Cytokines Increased number of Monocyte activated APCs migrating to the DLNs B-cell (naїve) B cell (memory) Enhancement and modulation of Antibody Plasma cell the adaptive response CD 4+ T cell Increased Monocyte uptake of Macrophage antigen by APCs CD 4+ T cell (diversity impacted) APC Adjuvant Cytokines (improved pattern) Major histocompatibility complex Illustrative figure based on concepts from: Pasquale AD, Preiss S, Da Silva FT et al. Vaccine adjuvants: from 1920 -2015 and beyond. Vaccines. 2015. 3: 320 -343. Garçon N, et al. Understanding modern vaccines, Perspectives in vaccinology, Vol 1, Amsterdam: Elsevier; 2011; Chapter 4: 89 -113 B-cell (naїve) Antibody. B cell (memory) Plasma cell Antigen B-cell (naїve) Antibody (wider profile)

The Future of Vaccines Safety considerations for the development of vaccines with novel adjuvants 15

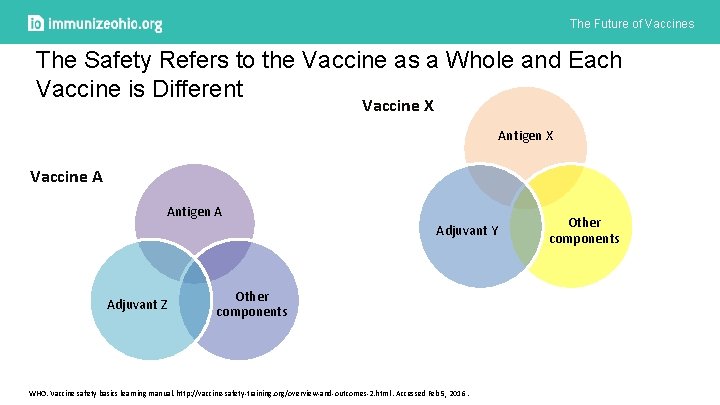
The Future of Vaccines The Safety Refers to the Vaccine as a Whole and Each Vaccine is Different Vaccine X Antigen X Vaccine A Antigen A Adjuvant Y Adjuvant Z Other components WHO. Vaccine safety basics learning manual. http: //vaccine-safety-training. org/overview-and-outcomes-2. html. Accessed Feb 5, 2016. Other components

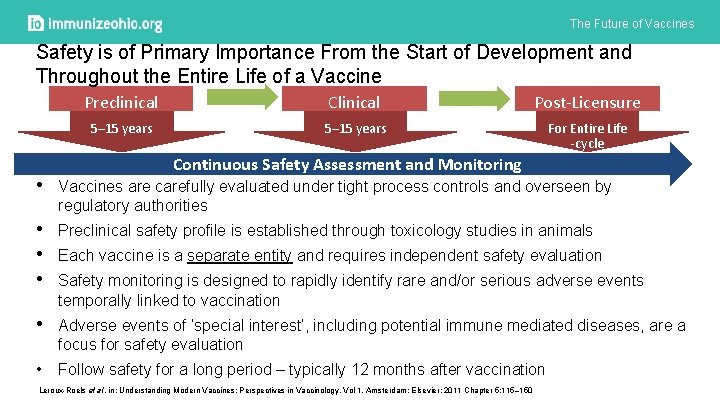
The Future of Vaccines Safety is of Primary Importance From the Start of Development and Throughout the Entire Life of a Vaccine Preclinical Clinical Post-Licensure 5– 15 years For Entire Life -cycle Continuous Safety Assessment and Monitoring • Vaccines are carefully evaluated under tight process controls and overseen by regulatory authorities • Preclinical safety profile is established through toxicology studies in animals • Each vaccine is a separate entity and requires independent safety evaluation • Safety monitoring is designed to rapidly identify rare and/or serious adverse events temporally linked to vaccination • Adverse events of ‘special interest’, including potential immune mediated diseases, are a focus for safety evaluation • Follow safety for a long period – typically 12 months after vaccination Leroux-Roels et al. in: Understanding Modern Vaccines: Perspectives in Vaccinology, Vol 1. Amsterdam: Elsevier; 2011 Chapter 5: 115– 150

The Future of Vaccines Adjuvanted Vaccines: General Reactogenicity and Safety Profile • • Adjuvanted vaccines often have increased reactogenicity, especially at the injection site Local symptoms are usually mild/moderate, short-lasting and do not impact compliance The safety profile of aluminium salt adjuvants has been well established through the use of billions of doses, in different populations, over more than 80 years Licensed, adjuvanted vaccines have clinically acceptable benefit-risk ratios Garçon N et al. Understanding Modern Vaccines: Perspectives in Vaccinology, Vol 1. Amsterdam: Elsevier; 2011 (Chapter 4: p 89– 113)

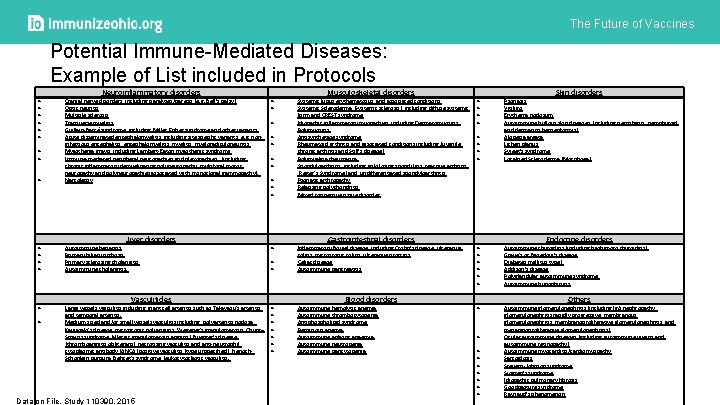
The Future of Vaccines Potential Immune-Mediated Diseases: Example of List included in Protocols Neuroinflammatory disorders Cranial nerve disorders, including paralyses/paresis (e. g. Bell’s palsy) Optic neuritis Multiple sclerosis Transverse myelitis Guillain-Barré syndrome, including Miller Fisher syndrome and other variants Acute disseminated encephalomyelitis, including site specific variants: e. g. noninfectious encephalitis, encephalomyelitis, myeloradiculoneuritis Myasthenia gravis, including Lambert-Eaton myasthenic syndrome Immune-mediated peripheral neuropathies and plexopathies, (including chronic inflammatory demyelinating polyneuropathy, multifocal motor neuropathy and polyneuropathies associated with monoclonal gammopathy). Narcolepsy Autoimmune hepatitis Primary biliary cirrhosis Primary sclerosing cholangitis Autoimmune cholangitis. Large vessels vasculitis including: giant cell arteritis such as Takayasu's arteritis and temporal arteritis. Medium sized and/or small vessels vasculitis including: polyarteritis nodosa, Kawasaki's disease, microscopic polyangiitis, Wegener's granulomatosis, Churg– Strauss syndrome (allergic granulomatous angiitis), Buerger’s disease (thromboangiitis obliterans), necrotizing vasculitis and anti-neutrophil cytoplasmic antibody (ANCA) positive vasculitis (type unspecified), Henoch. Schonlein purpura, Behcet's syndrome, leukocytoclastic vasculitis. Liver disorders Vasculitides Data on File. Study 110390. 2015 Musculoskeletal disorders Systemic lupus erythematosus and associated conditions Systemic Scleroderma (Systemic sclerosis), including diffuse systemic form and CREST syndrome Idiopathic inflammatory myopathies, including Dermatomyositis, Polymyositis, Antisynthetase syndrome Rheumatoid arthritis and associated conditions including Juvenile chronic arthritis and Still’s disease) Polymyalgia rheumatica Spondyloarthritis, including ankylosing spondylitis, reactive arthritis (Reiter's Syndrome) and undifferentiated spondyloarthritis Psoriatic arthropathy Relapsing polychondritis Mixed connective tissue disorder Gastrointestinal disorders Inflammatory Bowel disease, including Crohn’s disease, ulcerative colitis, microscopic colitis, ulcerative proctitis Celiac disease Autoimmune pancreatitis Autoimmune hemolytic anemia Autoimmune thrombocytopenia Antiphospholipid syndrome Pernicious anemia Autoimmune aplastic anaemia Autoimmune neutropenia Autoimmune pancytopenia Blood disorders Skin disorders Psoriasis Vitiligo Erythema nodosum Autoimmune bullous skin diseases (including pemphigus, pemphigoid and dermatitis herpetiformis) Alopecia areata Lichen planus Sweet’s syndrome Localised Scleroderma (Morphoea) Autoimmune thyroiditis (including Hashimoto thyroiditis) Grave's or Basedow’s disease Diabetes mellitus type I Addison’s disease Polyglandular autoimmune syndrome Autoimmune hypophysitis Autoimmune glomerulonephritis (including Ig. A nephropathy, glomerulonephritis rapidly progressive, membranous glomerulonephritis, membranoproliferative glomerulonephritis, and mesangioproliferative glomerulonephritis) Ocular autoimmune diseases (including autoimmune uveitis and autoimmune retinopathy) Autoimmune myocarditis/cardiomyopathy Sarcoidosis Stevens-Johnson syndrome Sjögren’s syndrome Idiopathic pulmonary fibrosis Goodpasture syndrome Raynaud’s phenomenon Endocrine disorders Others

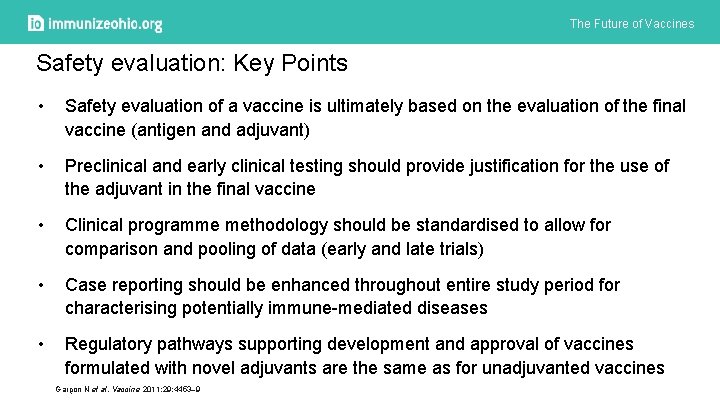
The Future of Vaccines Safety evaluation: Key Points • Safety evaluation of a vaccine is ultimately based on the evaluation of the final vaccine (antigen and adjuvant) • Preclinical and early clinical testing should provide justification for the use of the adjuvant in the final vaccine • Clinical programme methodology should be standardised to allow for comparison and pooling of data (early and late trials) • Case reporting should be enhanced throughout entire study period for characterising potentially immune-mediated diseases • Regulatory pathways supporting development and approval of vaccines formulated with novel adjuvants are the same as for unadjuvanted vaccines Garçon N et al. Vaccine 2011; 29: 4453– 9

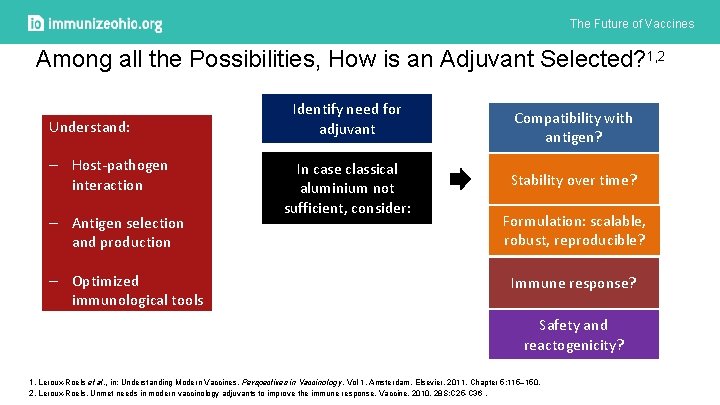
The Future of Vaccines Among all the Possibilities, How is an Adjuvant Selected? 1, 2 Understand: – Host-pathogen interaction – Antigen selection and production – Optimized immunological tools Identify need for adjuvant In case classical aluminium not sufficient, consider: Compatibility with antigen? Stability over time? Formulation: scalable, robust, reproducible? Immune response? Safety and reactogenicity? 1. Leroux-Roels et al. , in: Understanding Modern Vaccines, Perspectives in Vaccinology, Vol 1, Amsterdam, Elsevier, 2011, Chapter 5: 115– 150. 2. Leroux-Roels. Unmet needs in modern vaccinology adjuvants to improve the immune response. Vaccine. 2010. 28 S: C 25 -C 36.

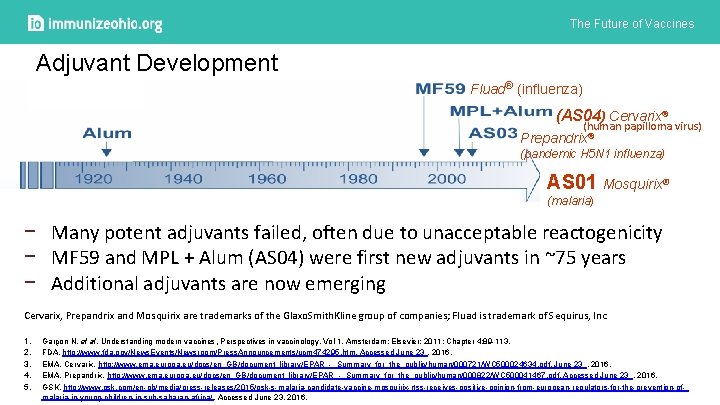
The Future of Vaccines Adjuvant Development Fluad® (influenza) (AS 04) Cervarix® (human papilloma virus) Prepandrix® (pandemic H 5 N 1 influenza) AS 01 Mosquirix® (malaria) − Many potent adjuvants failed, often due to unacceptable reactogenicity − MF 59 and MPL + Alum (AS 04) were first new adjuvants in ~75 years − Additional adjuvants are now emerging Cervarix, Prepandrix and Mosquirix are trademarks of the Glaxo. Smith. Kline group of companies; Fluad is trademark of Sequirus, Inc 1. 2. 3. 4. 5. Garçon N, et al. Understanding modern vaccines, Perspectives in vaccinology, Vol 1, Amsterdam: Elsevier; 2011; Chapter 4: 89 -113. FDA. http: //www. fda. gov/News. Events/Newsroom/Press. Announcements/ucm 474295. htm. Accessed June 23 , 2016. EMA. Cervarix. http: //www. ema. europa. eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000721/WC 500024634. pdf. June 23 , 2016. EMA. Prepandrix. http: //www. ema. europa. eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000822/WC 500041467. pdf. Accessed June 23 , 2016. GSK. http: //www. gsk. com/en-gb/media/press-releases/2015/gsk-s-malaria-candidate-vaccine-mosquirix-rtss-receives-positive-opinion-from-european-regulators-for-the-prevention-ofmalaria-in-young-children-in-sub-saharan-africa/. Accessed June 23, 2016.

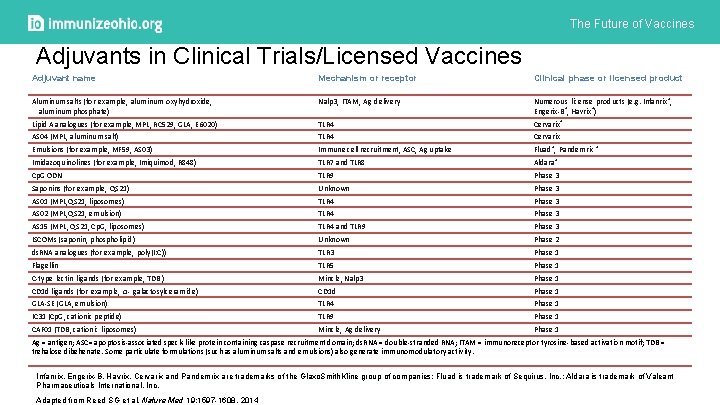
The Future of Vaccines Adjuvants in Clinical Trials/Licensed Vaccines Adjuvant name Mechanism or receptor Clinical phase or licensed product Aluminum salts (for example, aluminum oxyhydroxide, aluminum phosphate) Nalp 3, ITAM, Ag delivery Numerous license products (e. g. Infanrix®, Engerix-B®, Havrix®) Lipid A analogues (for example, MPL, RC 529, GLA, E 6020) TLR 4 Cervarix® AS 04 (MPL, aluminum salt) TLR 4 Cervarix Emulsions (for example, MF 59, AS 03) Immune cell recruitment, ASC, Ag uptake Fluad®, Pandemrix ® Imidazoquinolines (for example, Imiquimod, R 848) TLR 7 and TLR 8 Aldara® Cp. G ODN TLR 9 Phase 3 Saponins (for example, QS 21) Unknown Phase 3 AS 01 (MPL, QS 21, liposomes) TLR 4 Phase 3 AS 02 (MPL, QS 21, emulsion) TLR 4 Phase 3 AS 15 (MPL, QS 21, Cp. G, liposomes) TLR 4 and TLR 9 Phase 3 ISCOMs (saponin, phospholipid) Unknown Phase 2 ds. RNA analogues (for example, poly(I: C)) TLR 3 Phase 1 Flagellin TLR 5 Phase 1 C-type lectin ligands (for example, TDB ) Mincle, Nalp 3 Phase 1 CD 1 d ligands (for example, α- galactosylceramide) CD 1 d Phase 1 GLA-SE (GLA, emulsion) TLR 4 Phase 1 IC 31 (Cp. G, cationic peptide) TLR 9 Phase 1 CAF 01 (TDB, cationic liposomes) Mincle, Ag delivery Phase 1 Ag = antigen; ASC= apoptosis-associated speck-like protein containing caspase recruitment domain; ds. RNA = double-stranded RNA; ITAM = immunoreceptor tyrosine-based activation motif; TDB = trehalose dibehenate. Some particulate formulations (such as aluminum salts and emulsions) also generate immunomodulatory activity. Infanrix, Engerix-B, Havrix, Cervarix and Pandemrix are trademarks of the Glaxo. Smith. Kline group of companies; Fluad is trademark of Sequirus, Inc. ; Aldara is trademark of Valeant Pharmaceuticals International, Inc. Adapted from Reed SG et al, Nature Med 19: 1597 -1608, 2014

The Future of Vaccines GSK Adjuvant System (AS) Families

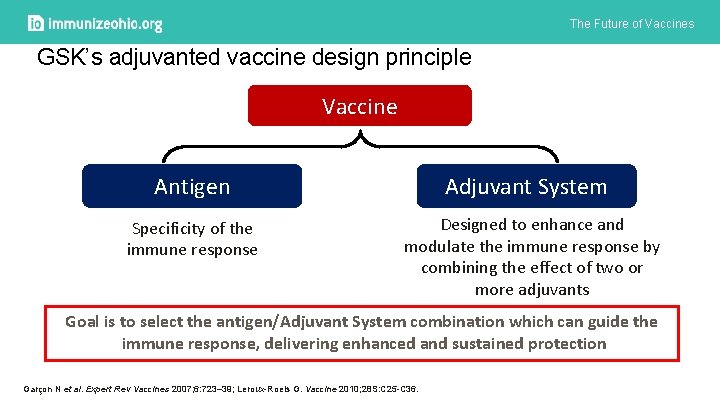
The Future of Vaccines GSK’s adjuvanted vaccine design principle Vaccine Antigen Specificity of the immune response Adjuvant System Designed to enhance and modulate the immune response by combining the effect of two or more adjuvants Goal is to select the antigen/Adjuvant System combination which can guide the immune response, delivering enhanced and sustained protection Garçon N et al. Expert Rev Vaccines 2007; 6: 723– 39; Leroux-Roels G. Vaccine 2010; 28 S: C 25 -C 36.

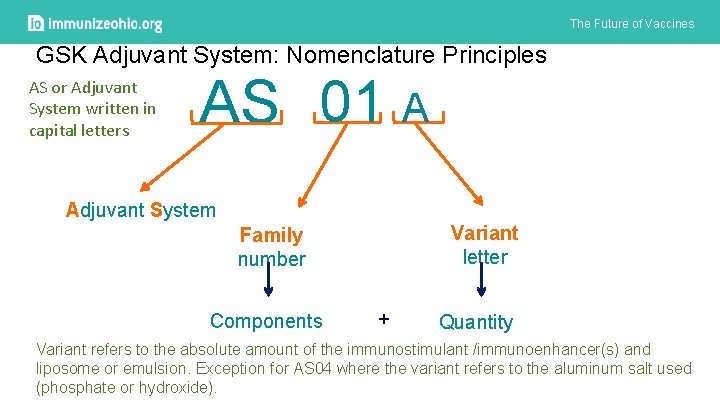
The Future of Vaccines GSK Adjuvant System: Nomenclature Principles AS or Adjuvant System written in capital letters AS 01 A Adjuvant System Variant letter Family number Components + Quantity Variant refers to the absolute amount of the immunostimulant /immunoenhancer(s) and liposome or emulsion. Exception for AS 04 where the variant refers to the aluminum salt used (phosphate or hydroxide).

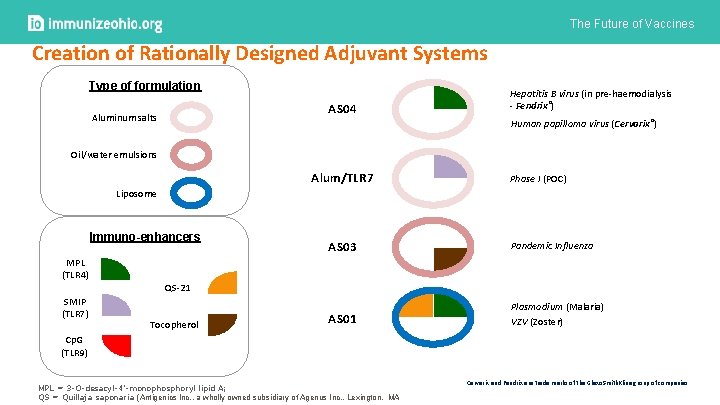
The Future of Vaccines Creation of Rationally Designed Adjuvant Systems Type of formulation AS 04 Aluminum salts Hepatitis B virus (in pre-haemodialysis - Fendrix®) Human papilloma virus (Cervarix®) Oil/water emulsions Alum/TLR 7 Phase I (POC) Liposome Immuno-enhancers MPL (TLR 4) SMIP (TLR 7) AS 03 Pandemic Influenza AS 01 Plasmodium (Malaria) VZV (Zoster) QS-21 Tocopherol Cp. G (TLR 9) MPL = 3 -O-desacyl-4’-monophosphoryl lipid A; QS = Quillaja saponaria (Antigenics Inc. , a wholly owned subsidiary of Agenus Inc. , Lexington, MA Cervarix and Fendrix are trade marks of the Glaxo. Smith. Kline group of companies.

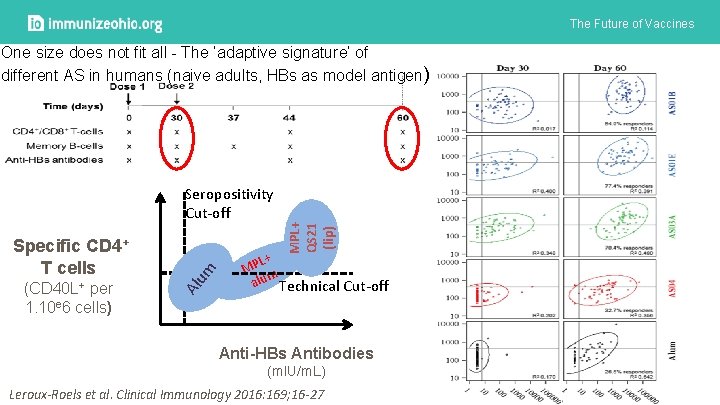
The Future of Vaccines Specific CD 4+ T cells (CD 40 L+ per 1. 10 e 6 cells) Al um Seropositivity Cut-off MPL+ QS 21 (lip) One size does not fit all - The ‘adaptive signature’ of different AS in humans (naive adults, HBs as model antigen) L+ MP m alu Technical Cut-off Anti-HBs Antibodies (m. IU/m. L) Leroux-Roels et al. Clinical Immunology 2016: 169; 16 -27

The Future of Vaccines GSK Adjuvant System: an historical perspective In-licensing of MPL, QS 21 and Cp. G Cohen J et al. Human Vaccines 2010; 6(1): 90 -96. Garçon et al. Expert Rev Vaccines 2007; 6: 723– 39. Garçon et al. Expert Rev Vaccines 2011; 10(4): 471 -486. RTS, S Clinical Trial Partnership. NEJM 2012; 367: 2284 -95. Lal H, et al. NEJM 2015; 372: 2087 -96 Zoster and Malaria phase III data

The Future of Vaccines Experience with investigational malaria vaccine

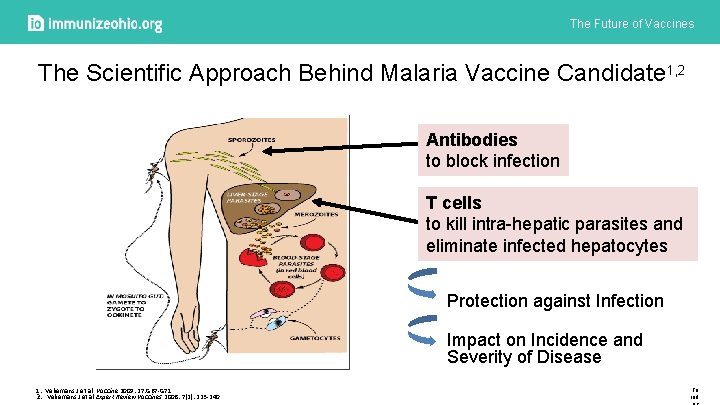
The Future of Vaccines The Scientific Approach Behind Malaria Vaccine Candidate 1, 2 Antibodies to block infection T cells to kill intra-hepatic parasites and eliminate infected hepatocytes Protection against Infection Impact on Incidence and Severity of Disease 1. Vekemans J et al. Vaccine 2009: 27: G 67 -G 71 2. Vekemans J et al Expert Review Vaccines 2008: 7(2): 223 -240 To ind

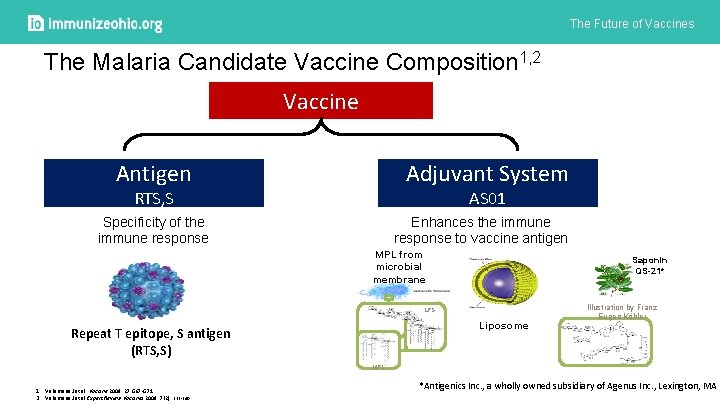
The Future of Vaccines The Malaria Candidate Vaccine Composition 1, 2 Vaccine Adjuvant System Antigen AS 01 RTS, S Specificity of the immune response Enhances the immune response to vaccine antigen MPL from microbial membrane Saponin QS-21* LPS Liposome Repeat T epitope, S antigen (RTS, S) Illustration by Franz Eugen Köhler MPL 1. Vekemans J et al. Vaccine 2009: 27: G 67 -G 71 2. Vekemans J et al Expert Review Vaccines 2008: 7(2): 223 -240 *Antigenics Inc. , a wholly owned subsidiary of Agenus Inc. , Lexington, MA

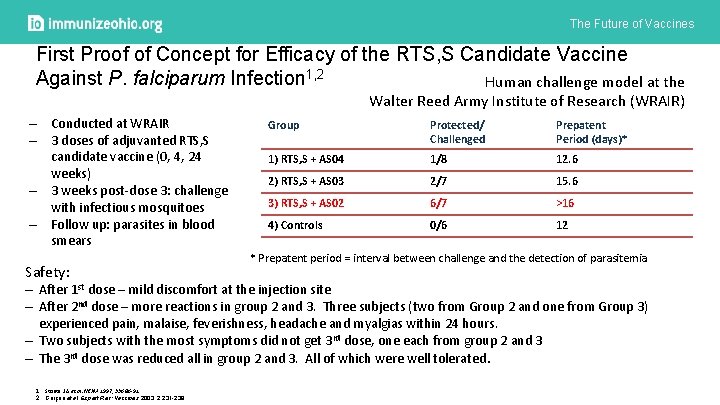
The Future of Vaccines First Proof of Concept for Efficacy of the RTS, S Candidate Vaccine Against P. falciparum Infection 1, 2 Human challenge model at the Walter Reed Army Institute of Research (WRAIR) – Conducted at WRAIR – 3 doses of adjuvanted RTS, S candidate vaccine (0, 4, 24 weeks) – 3 weeks post-dose 3: challenge with infectious mosquitoes – Follow up: parasites in blood smears Safety: Group Protected/ Challenged Prepatent Period (days)* 1) RTS, S + AS 04 1/8 12. 6 2) RTS, S + AS 03 2/7 15. 6 3) RTS, S + AS 02 6/7 >16 4) Controls 0/6 12 * Prepatent period = interval between challenge and the detection of parasitemia ‒ After 1 st dose – mild discomfort at the injection site ‒ After 2 nd dose – more reactions in group 2 and 3. Three subjects (two from Group 2 and one from Group 3) experienced pain, malaise, feverishness, headache and myalgias within 24 hours. ‒ Two subjects with the most symptoms did not get 3 rd dose, one each from group 2 and 3 ‒ The 3 rd dose was reduced all in group 2 and 3. All of which were well tolerated. 1. Stoute J A et al. NEJM 1997, 336: 86 -91 2. Garçon et al. Expert Rev: Vaccines 2003, 2: 231 -238

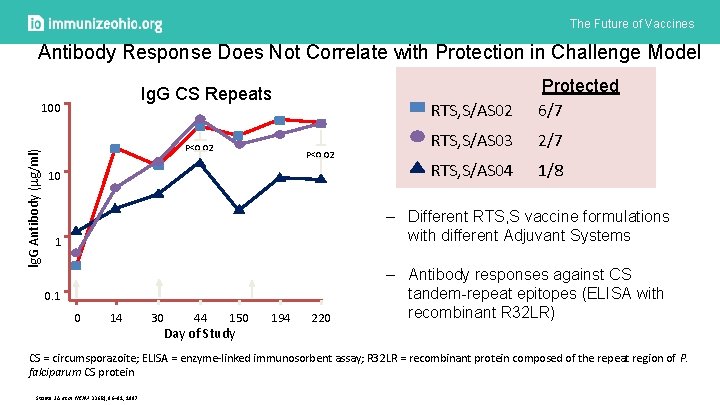
The Future of Vaccines Antibody Response Does Not Correlate with Protection in Challenge Model Ig. G CS Repeats lg. G Antibody ( g/ml) 100 P<0. 02 10 RTS, S/AS 02 Protected 6/7 RTS, S/AS 03 2/7 RTS, S/AS 04 1/8 – Different RTS, S vaccine formulations with different Adjuvant Systems 1 0 14 30 44 150 Day of Study 194 220 – Antibody responses against CS tandem-repeat epitopes (ELISA with recombinant R 32 LR) CS = circumsporazoite; ELISA = enzyme-linked immunosorbent assay; R 32 LR = recombinant protein composed of the repeat region of P. falciparum CS protein Stoute J A et al. NEJM 336(2), 86– 91, 1997

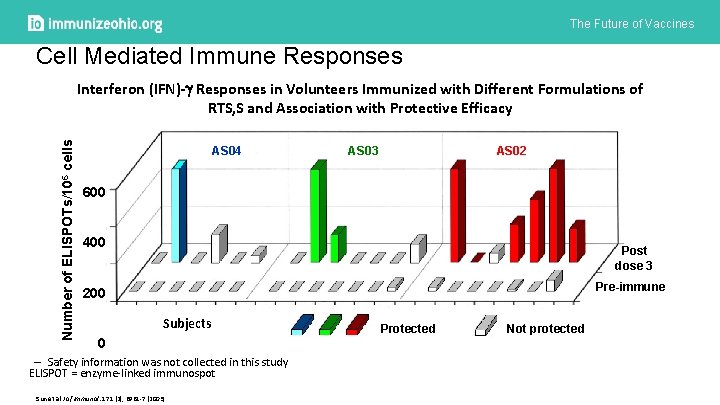
The Future of Vaccines Cell Mediated Immune Responses Number of ELISPOTs/106 cells Interferon (IFN)- Responses in Volunteers Immunized with Different Formulations of RTS, S and Association with Protective Efficacy AS 04 AS 03 AS 02 600 400 Post dose 3 Pre-immune 200 Subjects 0 – Safety information was not collected in this study ELISPOT = enzyme-linked immunospot Sun et al J of Immunol: 171 (2), 6961 -7 (2003) Protected Not protected

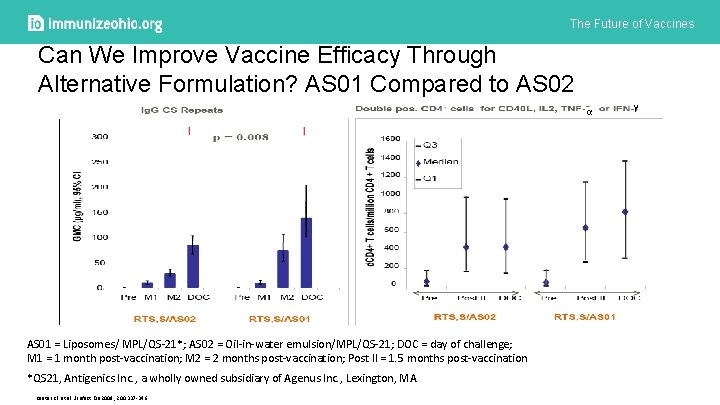
The Future of Vaccines Can We Improve Vaccine Efficacy Through Alternative Formulation? AS 01 Compared to AS 02 α AS 01 = Liposomes/ MPL/QS-21*; AS 02 = Oil-in-water emulsion/MPL/QS-21; DOC = day of challenge; M 1 = 1 month post-vaccination; M 2 = 2 months post-vaccination; Post II = 1. 5 months post-vaccination *QS 21, Antigenics Inc. , a wholly owned subsidiary of Agenus Inc. , Lexington, MA Kester KE et al. J Infect Dis 2009; 200: 337 -346. γ

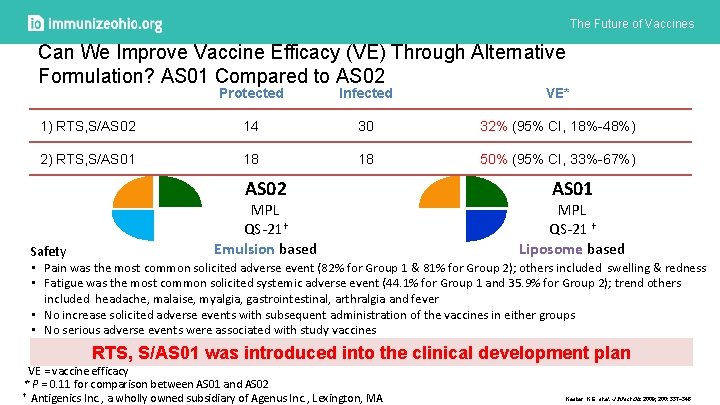
The Future of Vaccines Can We Improve Vaccine Efficacy (VE) Through Alternative Formulation? AS 01 Compared to AS 02 Protected Infected VE* 1) RTS, S/AS 02 14 30 32% (95% CI, 18%-48%) 2) RTS, S/AS 01 18 18 50% (95% CI, 33%-67%) AS 02 MPL QS-21† Emulsion based AS 01 MPL QS-21 † Liposome based Safety • Pain was the most common solicited adverse event (82% for Group 1 & 81% for Group 2); others included swelling & redness • Fatigue was the most common solicited systemic adverse event (44. 1% for Group 1 and 35. 9% for Group 2); trend others included headache, malaise, myalgia, gastrointestinal, arthralgia and fever • No increase solicited adverse events with subsequent administration of the vaccines in either groups • No serious adverse events were associated with study vaccines RTS, S/AS 01 was introduced into the clinical development plan VE = vaccine efficacy * P = 0. 11 for comparison between AS 01 and AS 02 † Antigenics Inc. , a wholly owned subsidiary of Agenus Inc. , Lexington, MA Kester K E et al. J Infect Dis 2009; 200: 337 -346

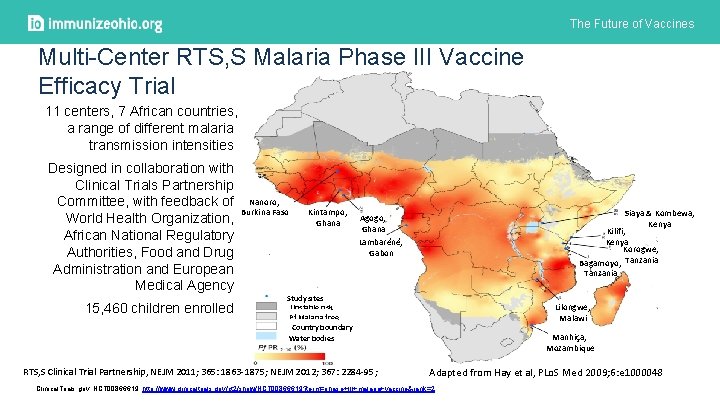
The Future of Vaccines Multi-Center RTS, S Malaria Phase III Vaccine Efficacy Trial 11 centers, 7 African countries, a range of different malaria transmission intensities Designed in collaboration with Clinical Trials Partnership Committee, with feedback of Nanoro, Burkina Faso World Health Organization, African National Regulatory Authorities, Food and Drug Administration and European Medical Agency 15, 460 children enrolled Kintampo, Ghana Siaya & Kombewa, Kenya Kilifi, Kenya Korogwe, Bagamoyo, Tanzania Agogo, Ghana Lambaréné, Gabon Study sites Unstable risk Pf Malaria free Country boundary Water bodies RTS, S Clinical Trial Partnership, NEJM 2011; 365: 1863 -1875; NEJM 2012; 367: 2284 -95; Lilongwe, Malawi Manhiça, Mozambique Adapted from Hay et al, PLo. S Med 2009; 6: e 1000048 Clinical. Trials. gov. NCT 00866619. http: //www. clinicaltrials. gov/ct 2/show/NCT 00866619? term=phase+III+malaria+vaccine&rank=2

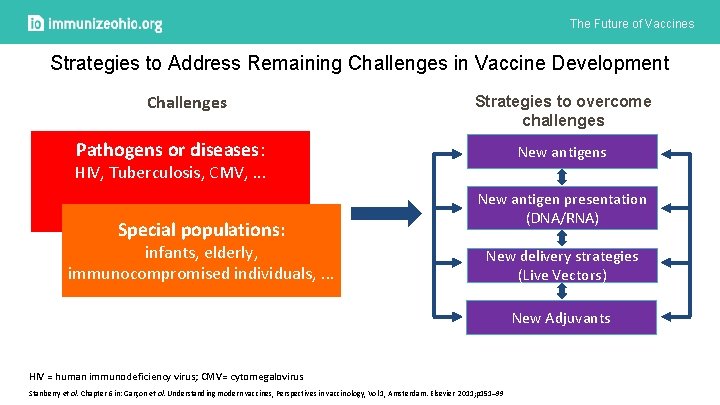
The Future of Vaccines Strategies to Address Remaining Challenges in Vaccine Development Challenges Strategies to overcome challenges Pathogens or diseases: New antigens HIV, Tuberculosis, CMV, . . . Special populations: infants, elderly, immunocompromised individuals, . . . New antigen presentation (DNA/RNA) New delivery strategies (Live Vectors) New Adjuvants HIV = human immunodeficiency virus; CMV= cytomegalovirus Stanberry et al. Chapter 6 in: Garçon et al. Understanding modern vaccines, Perspectives in vaccinology, Vol 1, Amsterdam. Elsevier 2011; p 151– 99

Va Sa ccin fe e ty Ne va w a cc nd ine o ld s Ad Va juv cc an ine te s d- log y ino cc Va “Vaccinology” adjuvants 40

The Future of Vaccines Vaccine science: two centuries of continuous research, improvements and achievements “Edward Jenner Vaccinating a Boy” EE Hillemacher, 1884. Courtesy of Wellcome Trust, Wellcome Library, London

The Future of Vaccines, today an explosion of new technologies 2015 Front Immunol. 2014; 5: 12. doi: 10. 3389/fimmu. 2014. 00012

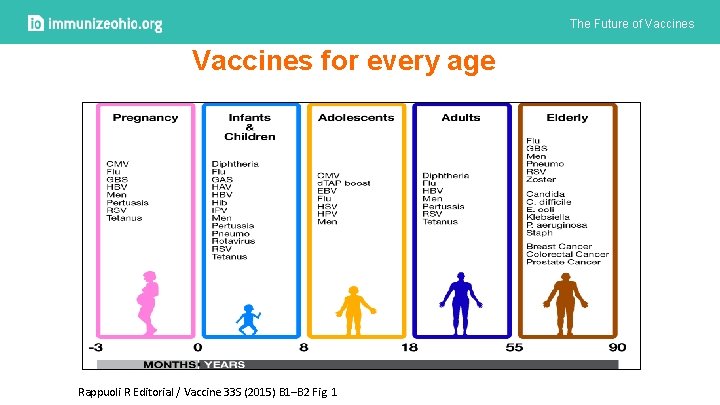
The Future of Vaccines for every age Rappuoli R Editorial / Vaccine 33 S (2015) B 1–B 2 Fig. 1

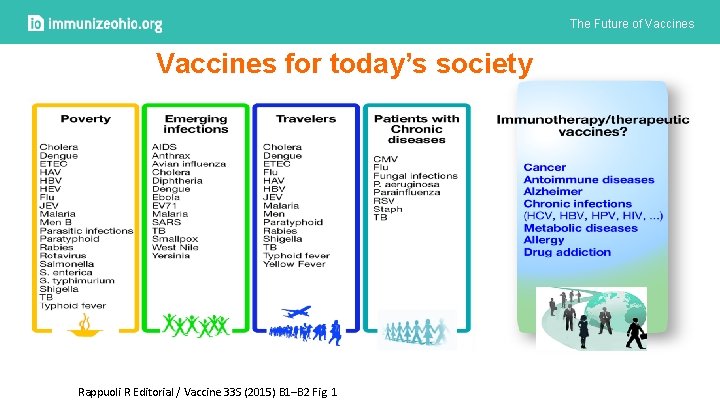
The Future of Vaccines for today’s society Rappuoli R Editorial / Vaccine 33 S (2015) B 1–B 2 Fig. 1

The Future of Vaccines Q&A

The Future of Vaccines Experience with investigational zoster vaccine

VZV T-cells Latent VZV infection and reactivation VZV primary infection induces VZV T-cell immune memory VZV immunity may be boosted periodically by exposure to varicella or silent reactivation from latency VZV-specific memory T-cells decline with age or in specific immune impairment conditions HZ threshold Varicella HZ Age → Immunity to HZ correlates with VZV T-cell levels HZ, herpes zoster; VZV, varicella zoster virus Kimberlin and Whitley. N Engl J Med 2007; 356: 1338 -43 CONFIDENTIAL INFORMATION – FOR INTERNAL USE ONLY The decline below a threshold correlates with an increased risk of HZ disease

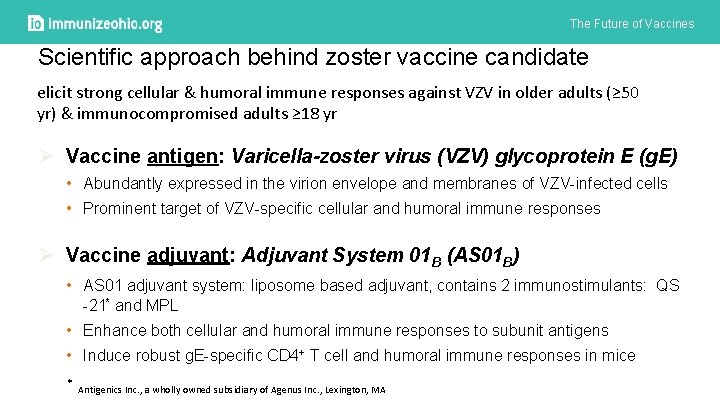
The Future of Vaccines Scientific approach behind zoster vaccine candidate elicit strong cellular & humoral immune responses against VZV in older adults (≥ 50 yr) & immunocompromised adults ≥ 18 yr Ø Vaccine antigen: Varicella-zoster virus (VZV) glycoprotein E (g. E) • Abundantly expressed in the virion envelope and membranes of VZV-infected cells • Prominent target of VZV-specific cellular and humoral immune responses Ø Vaccine adjuvant: Adjuvant System 01 B (AS 01 B) • AS 01 adjuvant system: liposome based adjuvant, contains 2 immunostimulants: QS -21* and MPL • Enhance both cellular and humoral immune responses to subunit antigens • Induce robust g. E-specific CD 4+ T cell and humoral immune responses in mice * Antigenics Inc. , a wholly owned subsidiary of Agenus Inc. , Lexington, MA

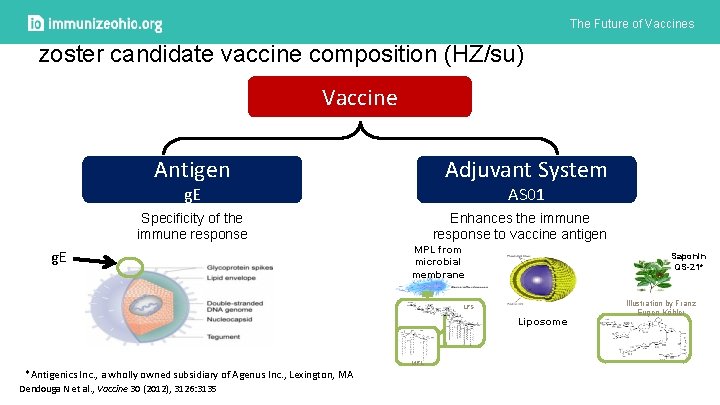
The Future of Vaccines zoster candidate vaccine composition (HZ/su) Vaccine Adjuvant System Antigen AS 01 g. E Specificity of the immune response g. E Enhances the immune response to vaccine antigen MPL from microbial membrane Saponin QS-21* LPS Liposome MPL *Antigenics Inc. , a wholly owned subsidiary of Agenus Inc. , Lexington, MA Dendouga N et al. , Vaccine 30 (2012), 3126: 3135 Illustration by Franz Eugen Köhler

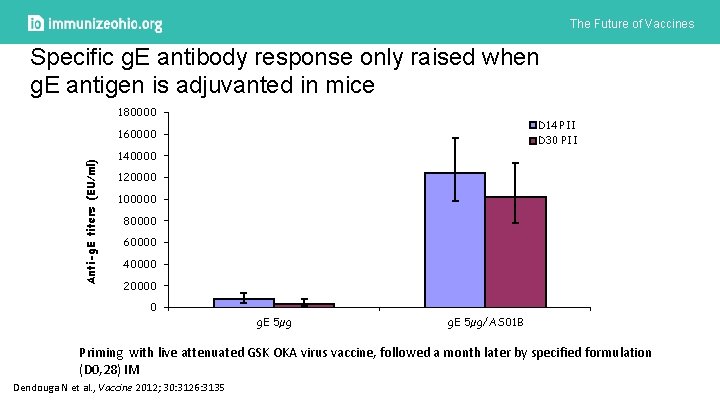
The Future of Vaccines Specific g. E antibody response only raised when g. E antigen is adjuvanted in mice 180000 D 14 PII Anti-g. E titers (EU/ml) 160000 D 30 PII 140000 120000 100000 80000 60000 40000 20000 0 g. E 5µg/AS 01 B Priming with live attenuated GSK OKA virus vaccine, followed a month later by specified formulation (D 0, 28) IM Dendouga N et al. , Vaccine 2012; 30: 3126: 3135

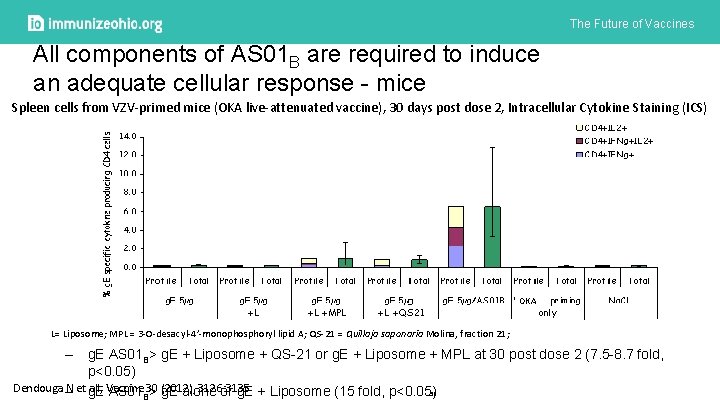
The Future of Vaccines All components of AS 01 B are required to induce an adequate cellular response - mice Spleen cells from VZV-primed mice (OKA live-attenuated vaccine), 30 days post dose 2, Intracellular Cytokine Staining (ICS) OKA L= Liposome; MPL = 3 -O-desacyl-4’-monophosphoryl lipid A; QS-21 = Quillaja saponaria Molina, fraction 21; – g. E AS 01 B> g. E + Liposome + QS-21 or g. E + Liposome + MPL at 30 post dose 2 (7. 5 -8. 7 fold, p<0. 05) Dendouga – N et g. E AS 01 all, Vaccine 30 (2012), 3126: 3135 51 B> g. E alone or g. E + Liposome (15 fold, p<0. 05)

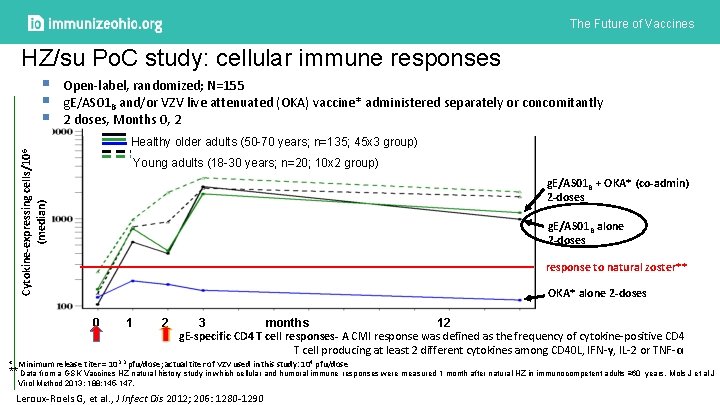
The Future of Vaccines HZ/su Po. C study: cellular immune responses Cytokine-expressing cells/106 (median) § § § Open-label, randomized; N=155 g. E/AS 01 B and/or VZV live attenuated (OKA) vaccine* administered separately or concomitantly 2 doses, Months 0, 2 Healthy older adults (50 -70 years; n=135; 45 x 3 group) Young adults (18 -30 years; n=20; 10 x 2 group) g. E/AS 01 B + OKA* (co-admin) 2 -doses g. E/AS 01 B alone 2 -doses response to natural zoster** OKA* alone 2 -doses 0 1 2 3 months 12 g. E-specific CD 4 T cell responses- A CMI response was defined as the frequency of cytokine-positive CD 4 T cell producing at least 2 different cytokines among CD 40 L, IFN-γ, IL-2 or TNF-α. * Minimum release titer = 103. 3 pfu/dose; actual titer of VZV used in this study: 104 pfu/dose ** Data from a GSK Vaccines HZ natural history study in which cellular and humoral immune responses were measured 1 month after natural HZ in immunocompetent adults ≥ 60 years. Mols J et al J Virol Method 2013; 188: 145 -147. Leroux-Roels G, et al. , J Infect Dis 2012; 206: 1280 -1290

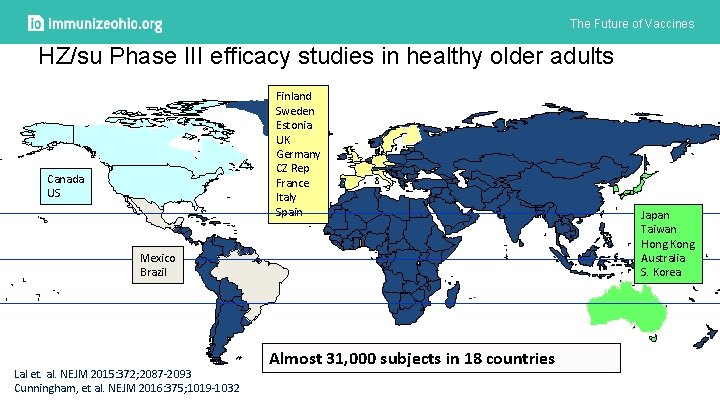
The Future of Vaccines HZ/su Phase III efficacy studies in healthy older adults Finland Sweden Estonia UK Germany CZ Rep France Italy Spain Canada US Mexico Brazil Lal et. al. NEJM 2015: 372; 2087 -2093 Cunningham, et al. NEJM 2016: 375; 1019 -1032 Almost 31, 000 subjects in 18 countries Japan Taiwan Hong Kong Australia S. Korea
 By the time future perfect
By the time future perfect Esercizi future continuous e future perfect
Esercizi future continuous e future perfect Advanced higher modern studies essay structure
Advanced higher modern studies essay structure Understanding standards advanced higher english
Understanding standards advanced higher english Diseases with vaccines
Diseases with vaccines Edible vaccines in pharmacognosy
Edible vaccines in pharmacognosy Edible vaccine definition
Edible vaccine definition Could vaccines breed viciousness
Could vaccines breed viciousness Www.cdc.gov/vaccines/schedules/index.html
Www.cdc.gov/vaccines/schedules/index.html Virulent
Virulent Stephanie seneff mit
Stephanie seneff mit Brighton collaboration criteria anaphylaxis
Brighton collaboration criteria anaphylaxis Edible vaccines pros and cons
Edible vaccines pros and cons Dna vaccines pros and cons
Dna vaccines pros and cons Freeze market
Freeze market Cancer vaccines
Cancer vaccines Spacing out vaccines
Spacing out vaccines Hep b vaccines
Hep b vaccines Glyphosate in vaccines
Glyphosate in vaccines Global alliance for vaccines and immunization
Global alliance for vaccines and immunization Tubertest idr
Tubertest idr Future perfect interrogative
Future perfect interrogative Present past and future continuous tense
Present past and future continuous tense Future perfect and future continuous
Future perfect and future continuous Present past future tense
Present past future tense Present continuous tense future plan
Present continuous tense future plan Future perfect future continuous exercises
Future perfect future continuous exercises Future continuous and future perfect objasnjenje
Future continuous and future perfect objasnjenje Tense chart
Tense chart Future nurse future midwife
Future nurse future midwife Future plans and finished future actions
Future plans and finished future actions điện thế nghỉ
điện thế nghỉ Dot
Dot Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ độ dài liên kết
độ dài liên kết Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Chó sói
Chó sói Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Một số thể thơ truyền thống
Một số thể thơ truyền thống Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Frameset trong html5
Frameset trong html5 Sơ đồ cơ thể người
Sơ đồ cơ thể người Số.nguyên tố
Số.nguyên tố đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton ưu thế lai là gì
ưu thế lai là gì Thẻ vin
Thẻ vin Tư thế ngồi viết
Tư thế ngồi viết Cái miệng xinh xinh thế chỉ nói điều hay thôi
Cái miệng xinh xinh thế chỉ nói điều hay thôi Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới