Unit 2 Informational Macromolecules Nucleic Acids Amino acids





- Slides: 12

Unit 2: Informational Macromolecules Nucleic Acids, Amino acids and protein structure

MACROMOLECULES • organized molecules that form the structure and carry out the activities of cells - Carbohydrates - Lipids - Proteins - Nucleic acids

CARBOHYDRATES • Monosaccharides: simple sugars = the building blocks • Oligosaccharides: 2 -10 sugar groups linked – These are often receptors for regulatory molecules • • Glycolipids (attached to lipids) and Glycoproteins (attached to proteins) • Polysaccharides: very long chains of sugars • i. e Glycogen

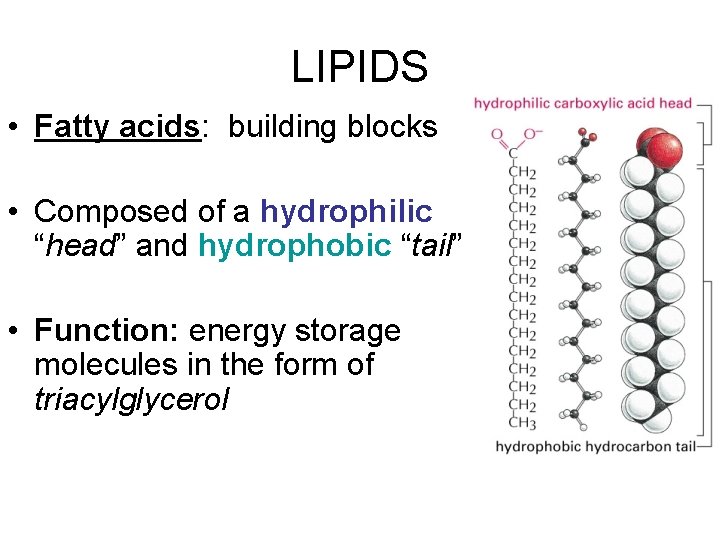
LIPIDS • Fatty acids: building blocks • Composed of a hydrophilic “head” and hydrophobic “tail” • Function: energy storage molecules in the form of triacylglycerol

PROTEINS • Amino acids = building blocks • 4 classes: – Basic – Polar - Acidic - Non-polar • Link together through peptide bonds to form the primary structure of a protein • H-bonding and folding lead to secondary and tertiary structure

Protein Structure Tertiary structure: • Side chain interaction determines how the protein will fold within itself. – i. e positively charged side chains might bind negatively charged side chains. • Changes to these amino acids can results in changes to protein folding and therefore affect function. Quaternary structure: proteins interacting with other proteins

Food for thought: When a protein-containing solution like milk is heated, a protein film forms on the surface. Why does it form?

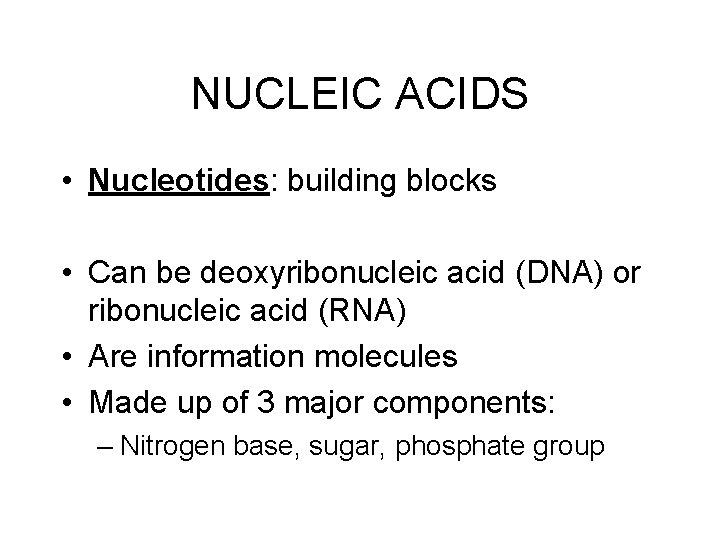
NUCLEIC ACIDS • Nucleotides: building blocks • Can be deoxyribonucleic acid (DNA) or ribonucleic acid (RNA) • Are information molecules • Made up of 3 major components: – Nitrogen base, sugar, phosphate group

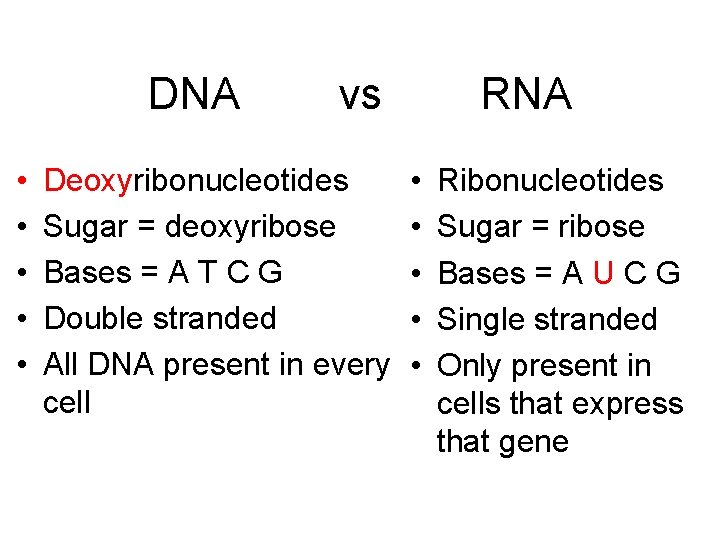
DNA • • • vs Deoxyribonucleotides Sugar = deoxyribose Bases = A T C G Double stranded All DNA present in every cell RNA • • • Ribonucleotides Sugar = ribose Bases = A U C G Single stranded Only present in cells that express that gene

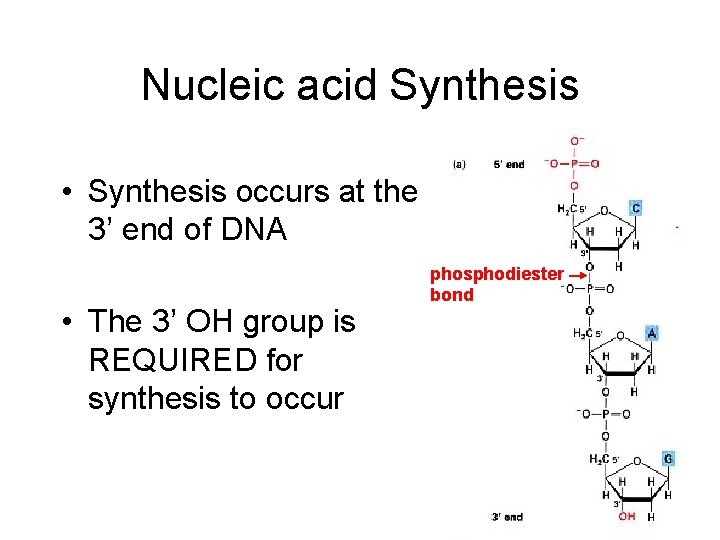
Nucleic acid Synthesis • Synthesis occurs at the 3’ end of DNA • The 3’ OH group is REQUIRED for synthesis to occur phosphodiester bond

Hyrdogen bonding • Hydrogen bonds are between complimentary pairs of purines and pyrimidines • Hold together the TWO strands of DNA • A-T = 2 hydrogen bonds • G-C = 3 hydrogen bonds WHY IS THIS IMPORTANT?

Practice Question Which of the two double-stranded DNA molecules shown below will be most resistant to the effects of heating A. 5’-AGCAGTTCATTATTCTCTCGTCGA -3’ 3’-TCGTCAAGTAATAAGAGAGCAGCA-5’ or B. 5’-TCCTCGAGCCTCCTGCGCCGCCGA -3’ 3’-AGGAGCTCGGAGGACGCGGCGGCT-5’ Why?