UMassAmherst Polymer Science Engineering Honors 391 D Section




- Slides: 29

UMass-Amherst Polymer Science & Engineering Honors 391 D Section 6 “Why is Renewable Energy not a Reality Today” E. Bryan Coughlin Polymer Science and Engineering UMass Amherst Coughlin@mail. pse. umass. edu Conte A 612 ext. 7 -1616

UMass-Amherst Polymer Science & Engineering Today’s Discussion Topics “Why Isn’t There a Hydrogen Economy? ” and “Will There Ever Be a Hydrogen Economy? ” August 24 Article on Physorg. com http: //www. physorg. com/news 170326193. html

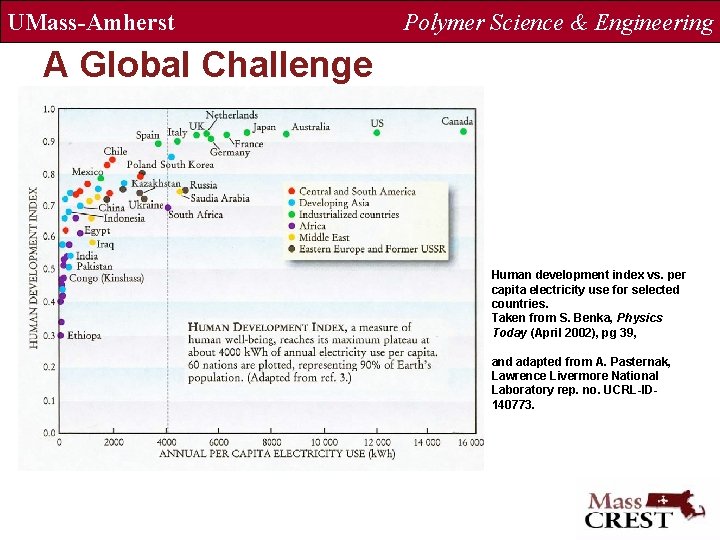
UMass-Amherst Polymer Science & Engineering A Global Challenge Human development index vs. per capita electricity use for selected countries. Taken from S. Benka, Physics Today (April 2002), pg 39, and adapted from A. Pasternak, Lawrence Livermore National Laboratory rep. no. UCRL-ID 140773.

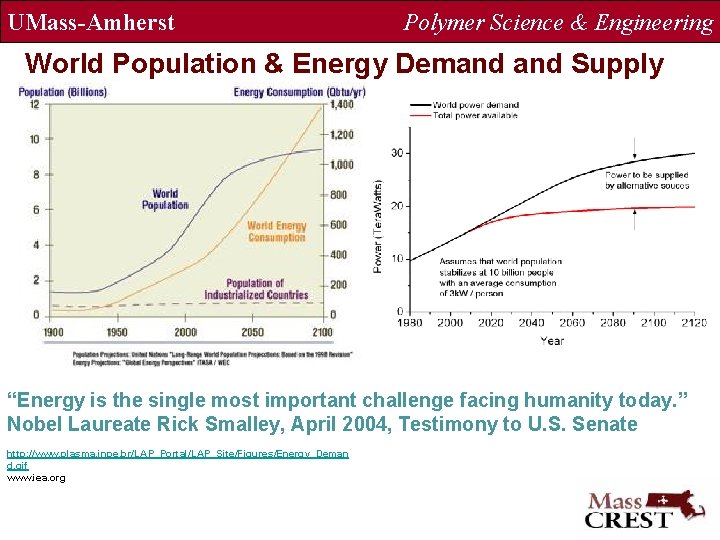
UMass-Amherst Polymer Science & Engineering World Population & Energy Demand Supply “Energy is the single most important challenge facing humanity today. ” Nobel Laureate Rick Smalley, April 2004, Testimony to U. S. Senate http: //www. plasma. inpe. br/LAP_Portal/LAP_Site/Figures/Energy_Deman d. gif www. iea. org

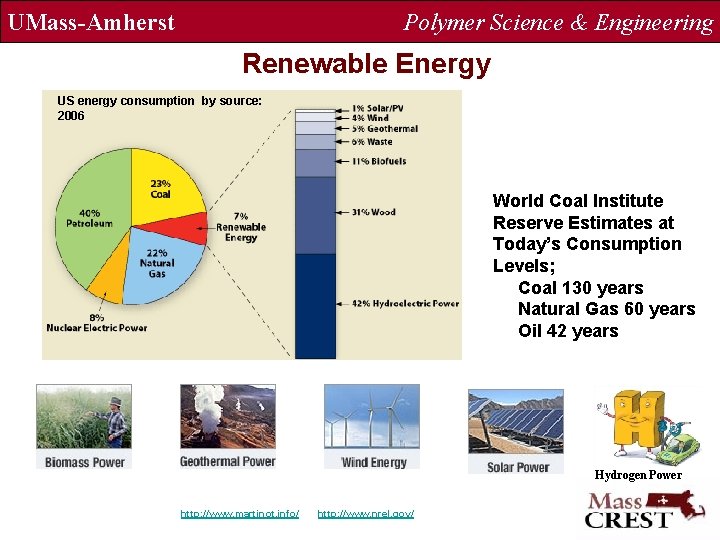
UMass-Amherst Polymer Science & Engineering Renewable Energy US energy consumption by source: 2006 World Coal Institute Reserve Estimates at Today’s Consumption Levels; Coal 130 years Natural Gas 60 years Oil 42 years Hydrogen Power http: //www. martinot. info/ http: //www. nrel. gov/

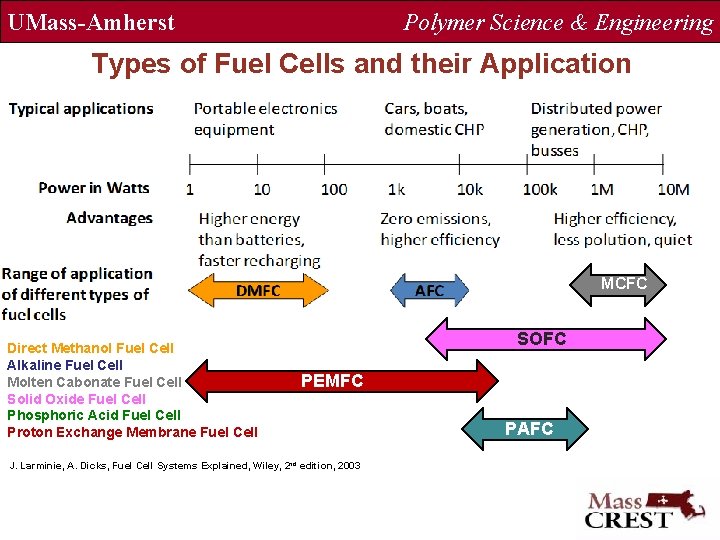
UMass-Amherst Polymer Science & Engineering Types of Fuel Cells and their Application MCFC Direct Methanol Fuel Cell Alkaline Fuel Cell Molten Cabonate Fuel Cell Solid Oxide Fuel Cell Phosphoric Acid Fuel Cell Proton Exchange Membrane Fuel Cell SOFC PEMFC J. Larminie, A. Dicks, Fuel Cell Systems Explained, Wiley, 2 nd edition, 2003 PAFC

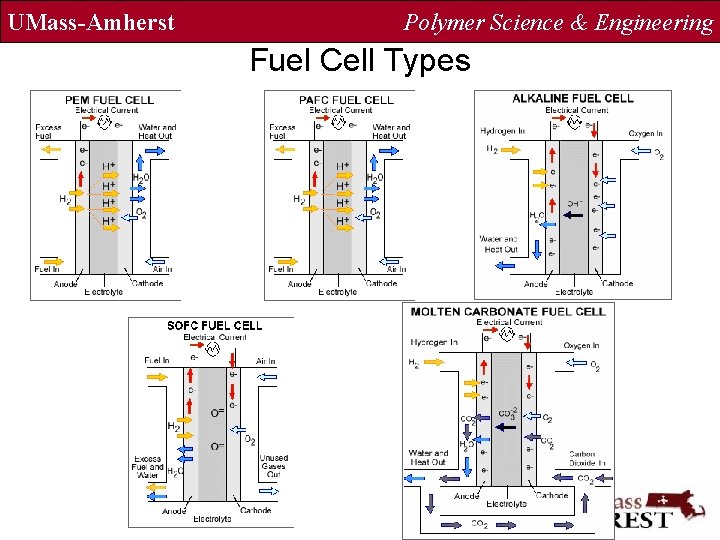
UMass-Amherst Polymer Science & Engineering Fuel Cell Types

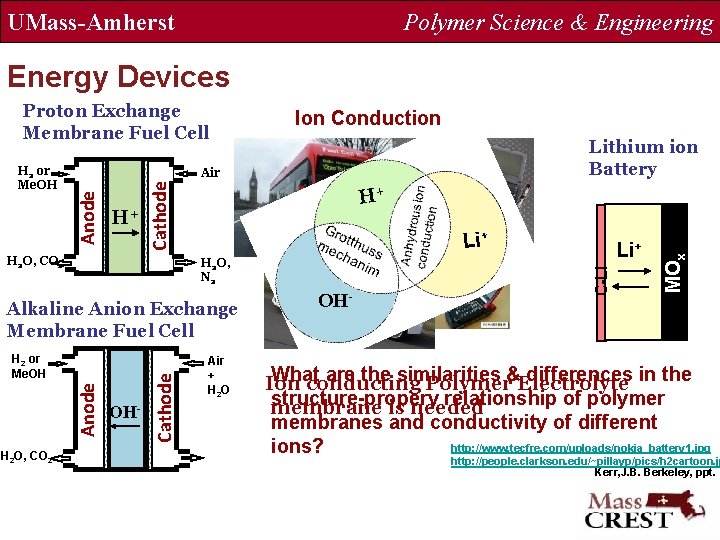
UMass-Amherst Polymer Science & Engineering Energy Devices Proton Exchange Membrane Fuel Cell Cathode Li+ H 2 O, N 2 Anode H 2 or Me. OH OH- Cathode Alkaline Anion Exchange Membrane Fuel Cell Air + H 2 O OH- Li+ MOx H+ C-Li H+ H 2 O, CO 2 Lithium ion Battery Air Anode H 2 or Me. OH Ion Conduction What are the similarities differences in the Ion conducting Polymer&Electrolyte structure-propery relationship of polymer membrane is needed membranes and conductivity of different http: //www. tecfre. com/uploads/nokia_battery 1. jpg ions? http: //people. clarkson. edu/~pillayp/pics/h 2 cartoon. jp Kerr, J. B. Berkeley, ppt.

UMass-Amherst Polymer Science & Engineering Why fuel cells? Polymer electrolyte fuel cells (PEMFC’s) can be used as highly efficient, non polluting power sources for vehicles and electronic devices. PEMFC’s Advantages: Low operation temperatures High power and energy density Different fuel sources (commonly hydrogen or methanol) may be employed Portable energy Highly efficient energy conversion (35% for PEMFC’s compared to ~15% for internal combustion engines) For a review see: Whittingham, M. S, Savinell, R. F. , Zawodzinski, T (editors), Chem. Rev. , 2004, 10.

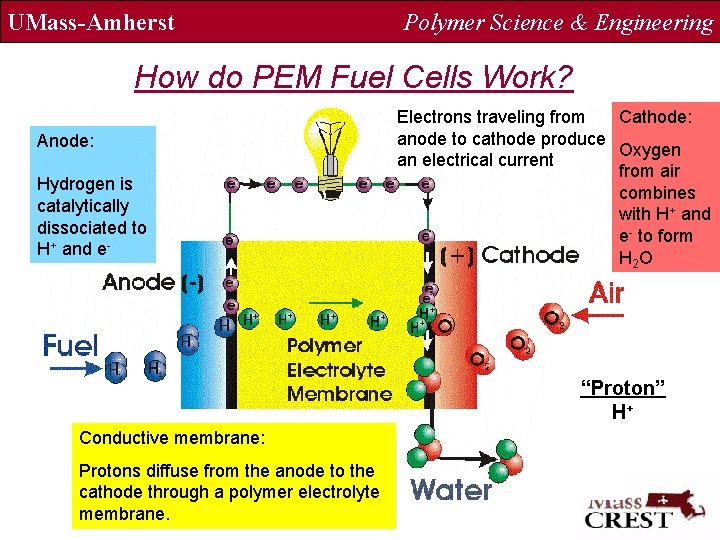
UMass-Amherst Polymer Science & Engineering How do PEM Fuel Cells Work? Anode: Hydrogen is catalytically dissociated to H+ and e- Electrons traveling from Cathode: anode to cathode produce Oxygen an electrical current from air combines with H+ and e- to form H 2 O “Proton” H+ Conductive membrane: Protons diffuse from the anode to the cathode through a polymer electrolyte membrane.

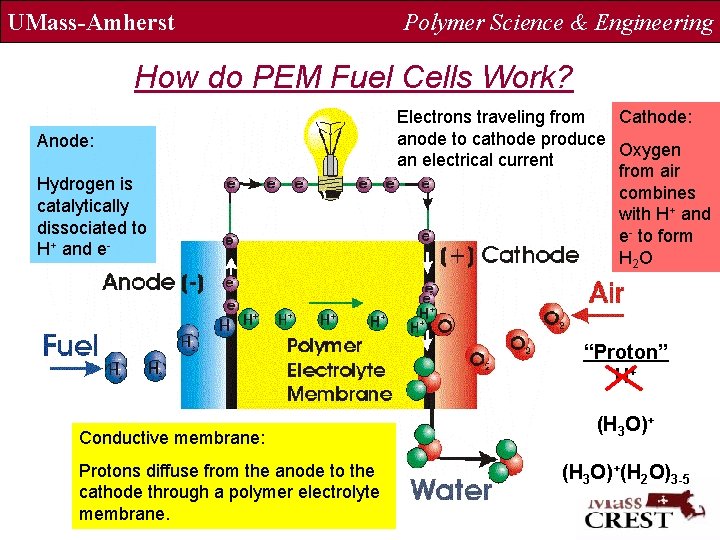
UMass-Amherst Polymer Science & Engineering How do PEM Fuel Cells Work? Anode: Hydrogen is catalytically dissociated to H+ and e- Electrons traveling from Cathode: anode to cathode produce Oxygen an electrical current from air combines with H+ and e- to form H 2 O “Proton” H+ Conductive membrane: Protons diffuse from the anode to the cathode through a polymer electrolyte membrane. (H 3 O)+(H 2 O)3 -5

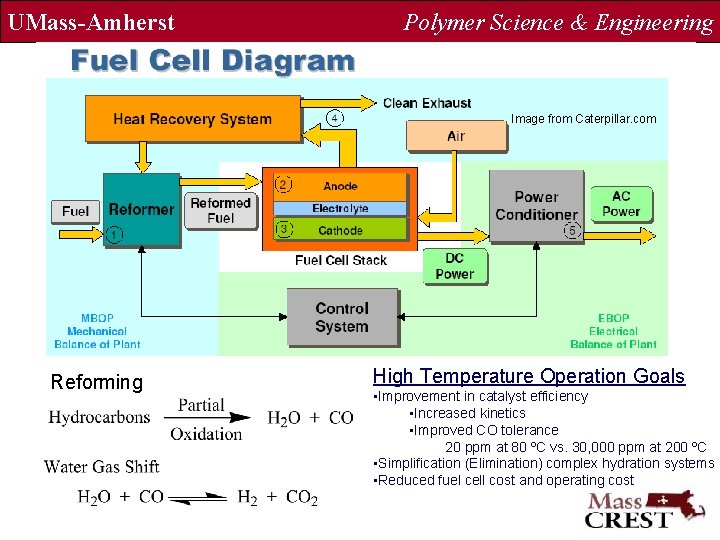
UMass-Amherst Polymer Science & Engineering Image from Caterpillar. com Reforming High Temperature Operation Goals • Improvement in catalyst efficiency • Increased kinetics • Improved CO tolerance 20 ppm at 80 ºC vs. 30, 000 ppm at 200 ºC • Simplification (Elimination) complex hydration systems • Reduced fuel cell cost and operating cost

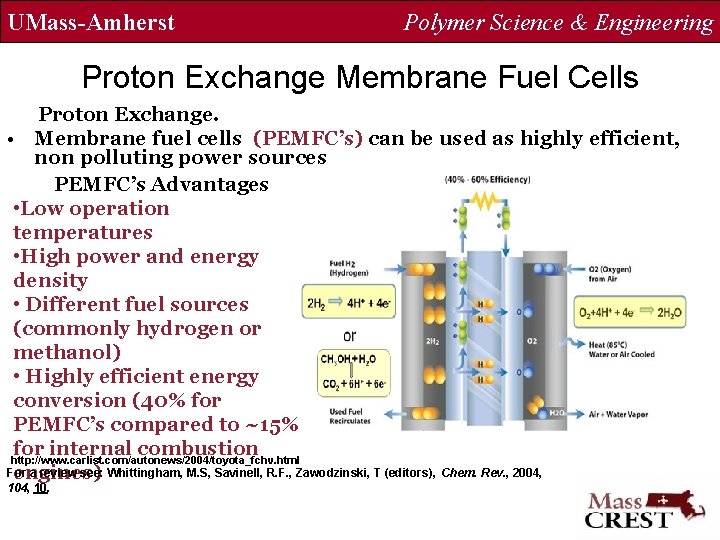
UMass-Amherst Polymer Science & Engineering Proton Exchange Membrane Fuel Cells Proton Exchange. • Membrane fuel cells (PEMFC’s) can be used as highly efficient, non polluting power sources PEMFC’s Advantages • Low operation temperatures • High power and energy density • Different fuel sources (commonly hydrogen or methanol) • Highly efficient energy conversion (40% for PEMFC’s compared to ~15% for internal combustion http: //www. carlist. com/autonews/2004/toyota_fchv. html For a review see: Whittingham, M. S, Savinell, R. F. , Zawodzinski, T (editors), Chem. Rev. , 2004, engines) 104, 10.

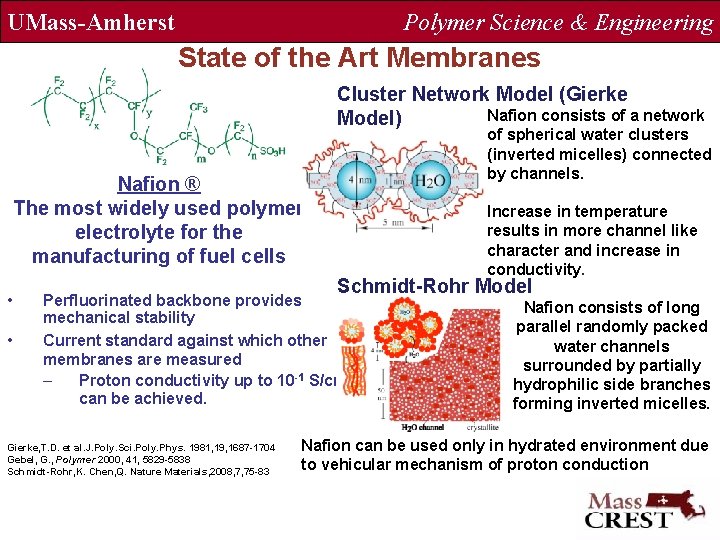
UMass-Amherst Polymer Science & Engineering State of the Art Membranes Cluster Network Model (Gierke Nafion consists of a network Model) of spherical water clusters (inverted micelles) connected by channels. Nafion ® The most widely used polymer electrolyte for the manufacturing of fuel cells • • Schmidt-Rohr Model Perfluorinated backbone provides mechanical stability Current standard against which other membranes are measured – Proton conductivity up to 10 -1 S/cm can be achieved. Gierke, T. D. et al. J. Poly. Sci. Poly. Phys. 1981, 19, 1687 -1704 Gebel, G. , Polymer 2000, 41, 5829 -5838 Schmidt-Rohr, K. Chen, Q. Nature Materials, 2008, 7, 75 -83 Increase in temperature results in more channel like character and increase in conductivity. Nafion consists of long parallel randomly packed water channels surrounded by partially hydrophilic side branches forming inverted micelles. Nafion can be used only in hydrated environment due to vehicular mechanism of proton conduction

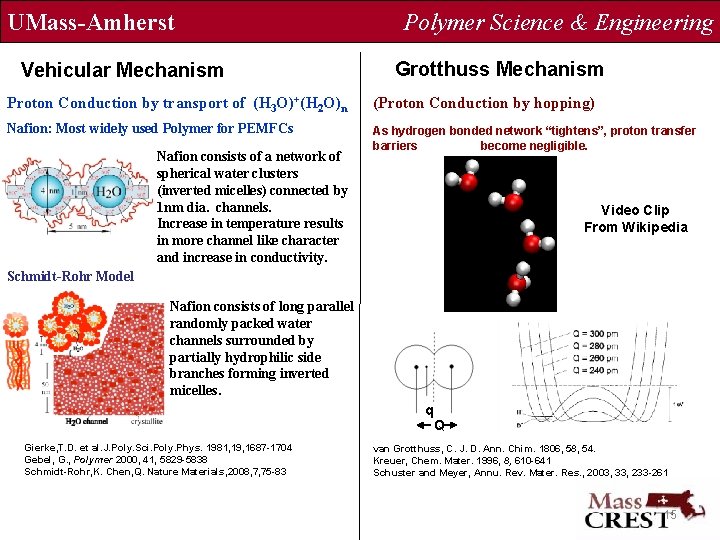
UMass-Amherst Vehicular Mechanism Polymer Science & Engineering Grotthuss Mechanism Proton Conduction by transport of (H 3 O)+(H 2 O)n (Proton Conduction by hopping) Nafion: Most widely used Polymer for PEMFCs As hydrogen bonded network “tightens”, proton transfer barriers become negligible. Nafion consists of a network of spherical water clusters (inverted micelles) connected by 1 nm dia. channels. Increase in temperature results in more channel like character and increase in conductivity. Video Clip From Wikipedia Schmidt-Rohr Model Nafion consists of long parallel randomly packed water channels surrounded by partially hydrophilic side branches forming inverted micelles. q Gierke, T. D. et al. J. Poly. Sci. Poly. Phys. 1981, 19, 1687 -1704 Gebel, G. , Polymer 2000, 41, 5829 -5838 Schmidt-Rohr, K. Chen, Q. Nature Materials, 2008, 7, 75 -83 Q van Grotthuss, C. J. D. Ann. Chim. 1806, 58, 54. Kreuer, Chem. Mater. 1996, 8, 610 -641 Schuster and Meyer, Annu. Rev. Mater. Res. , 2003, 33, 233 -261 15

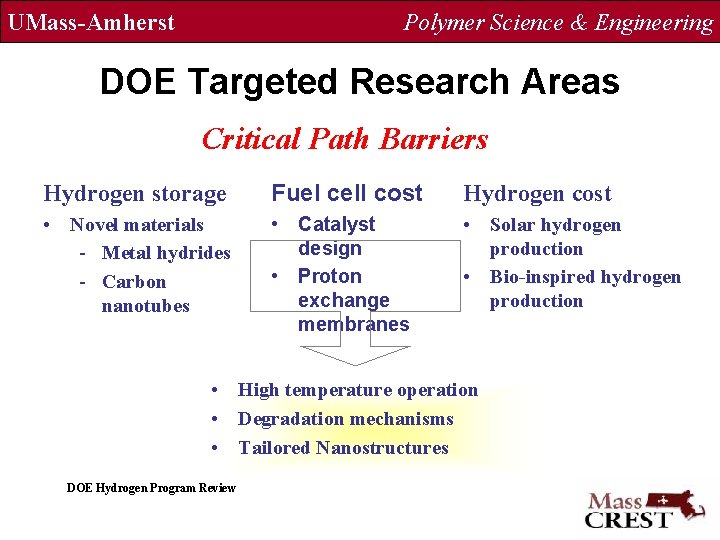
UMass-Amherst Polymer Science & Engineering DOE Targeted Research Areas Critical Path Barriers Hydrogen storage Fuel cell cost Hydrogen cost • Novel materials - Metal hydrides - Carbon nanotubes • Catalyst design • Proton exchange membranes • Solar hydrogen production • Bio-inspired hydrogen production • High temperature operation • Degradation mechanisms • Tailored Nanostructures DOE Hydrogen Program Review

UMass-Amherst Polymer Science & Engineering

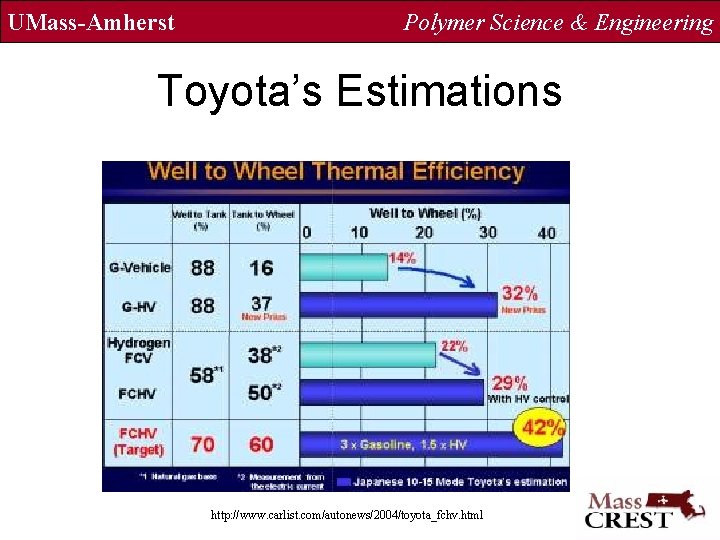
UMass-Amherst Polymer Science & Engineering Toyota’s Estimations http: //www. carlist. com/autonews/2004/toyota_fchv. html

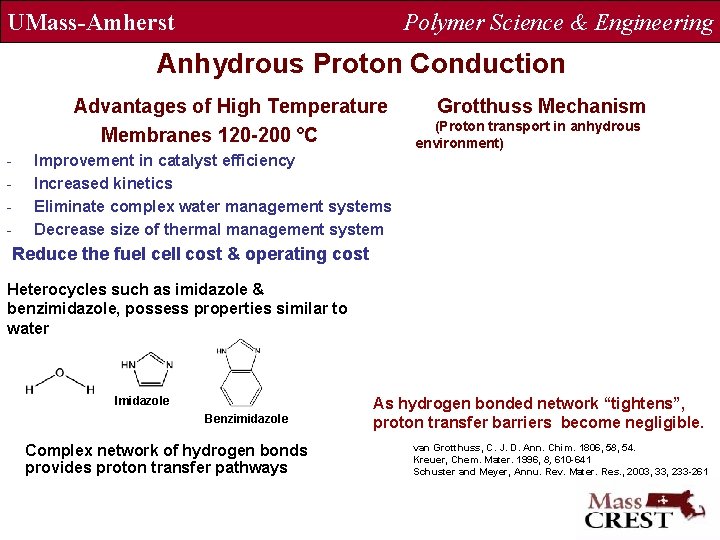
UMass-Amherst Polymer Science & Engineering Anhydrous Proton Conduction Advantages of High Temperature Membranes 120 -200 °C - Grotthuss Mechanism (Proton transport in anhydrous environment) Improvement in catalyst efficiency Increased kinetics Eliminate complex water management systems Decrease size of thermal management system Reduce the fuel cell cost & operating cost Heterocycles such as imidazole & benzimidazole, possess properties similar to water Imidazole Benzimidazole Complex network of hydrogen bonds provides proton transfer pathways As hydrogen bonded network “tightens”, proton transfer barriers become negligible. van Grotthuss, C. J. D. Ann. Chim. 1806, 58, 54. Kreuer, Chem. Mater. 1996, 8, 610 -641 Schuster and Meyer, Annu. Rev. Mater. Res. , 2003, 33, 233 -261

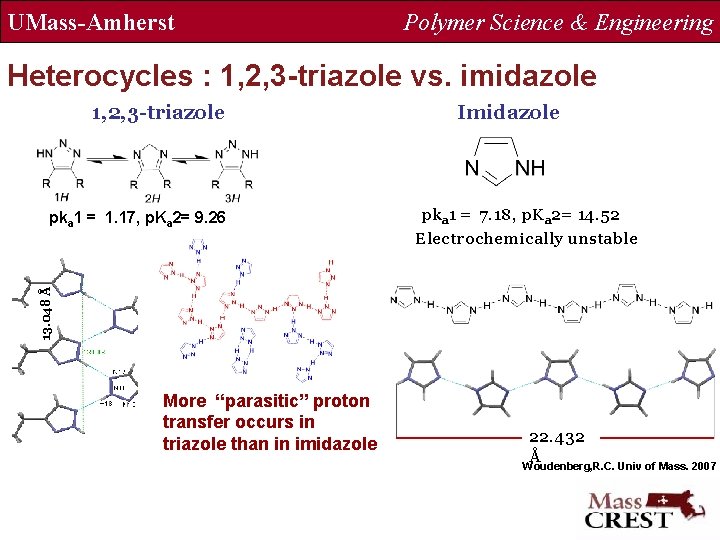
UMass-Amherst Polymer Science & Engineering Heterocycles : 1, 2, 3 -triazole vs. imidazole 1, 2, 3 -triazole pka 1 = 7. 18, p. Ka 2= 14. 52 Electrochemically unstable 13. 048 Å pka 1 = 1. 17, p. Ka 2= 9. 26 Imidazole More “parasitic” proton transfer occurs in triazole than in imidazole 22. 432 Å Woudenberg, R. C. Univ of Mass. 2007

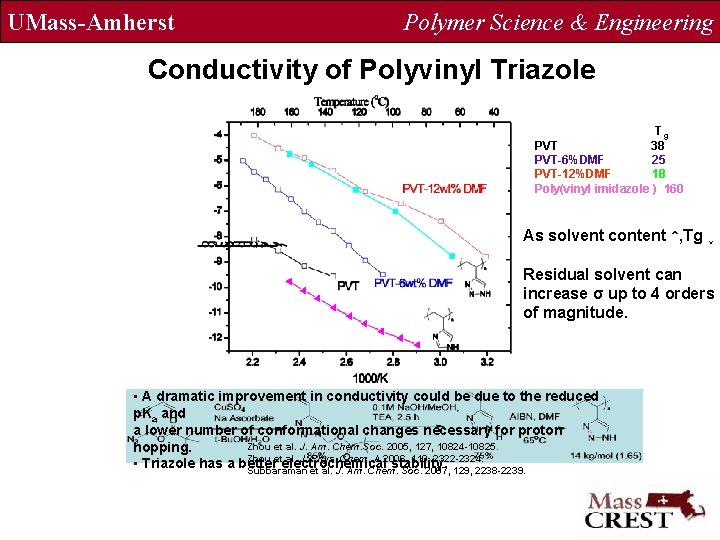
UMass-Amherst Polymer Science & Engineering Conductivity of Polyvinyl Triazole Tg PVT 38 PVT-6%DMF 25 PVT-12%DMF 18 Poly(vinyl imidazole ) 160 As solvent content ↑, Tg ↓ Residual solvent can increase σ up to 4 orders of magnitude. • A dramatic improvement in conductivity could be due to the reduced p. Ka and a lower number of conformational changes necessary for proton Zhou et al. J. Am. Chem. Soc. 2005, 127, 10824 -10825. hopping. Zhou et al. J. Phys. Chem. A 2006, 110, 2322 -2324. • Triazole has a better electrochemical stability. Subbaraman et al. J. Am. Chem. Soc. 2007, 129, 2238 -2239.

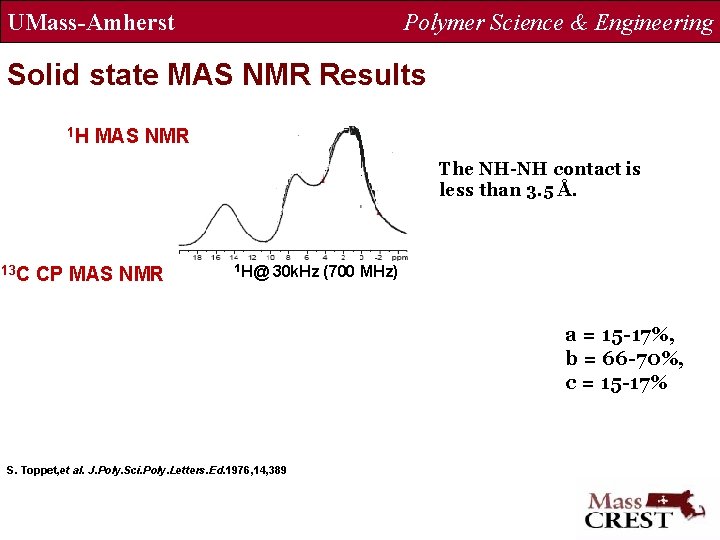
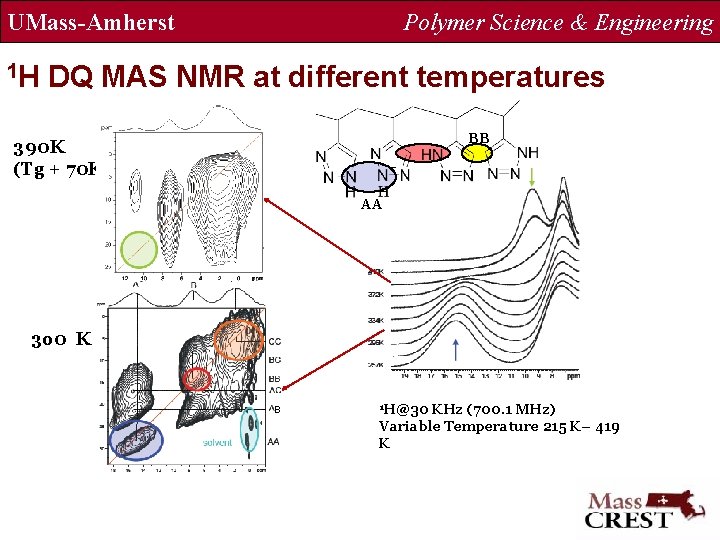
UMass-Amherst Polymer Science & Engineering Solid state MAS NMR Results 1 H MAS NMR The NH-NH contact is less than 3. 5 Å. 13 C CP MAS NMR 1 H@ 30 k. Hz (700 MHz) a = 15 -17%, b = 66 -70%, c = 15 -17% S. Toppet, et al. J. Poly. Sci. Poly. Letters. Ed. 1976, 14, 389

UMass-Amherst 1 H Polymer Science & Engineering DQ MAS NMR at different temperatures BB 390 K (Tg + 70 K) AA 3 o 0 K B 1 H@30 KHz (700. 1 MHz) Variable Temperature 215 K– 419 K

UMass-Amherst Polymer Science & Engineering Acknowledgement Royal Thai Government (fellowship for S. M. ) UMASS MRSEC on Polymers

UMass-Amherst Polymer Science & Engineering Today’s Discussion Topics “Why Isn’t There a Hydrogen Economy? ” and “Will There Ever Be a Hydrogen Economy? ” August 24 Article on Physorg. com http: //www. physorg. com/news 170326193. html

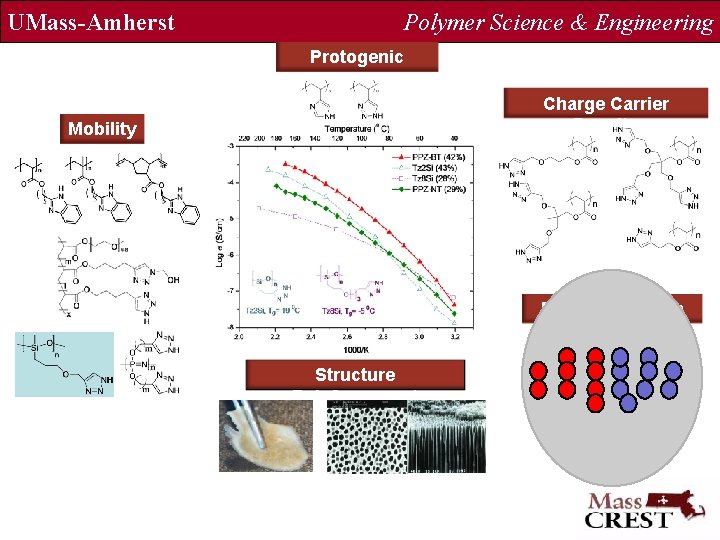
UMass-Amherst Polymer Science & Engineering Protogenic Group Charge Carrier Density Mobility Dual Mechanism Structure Reinforcement

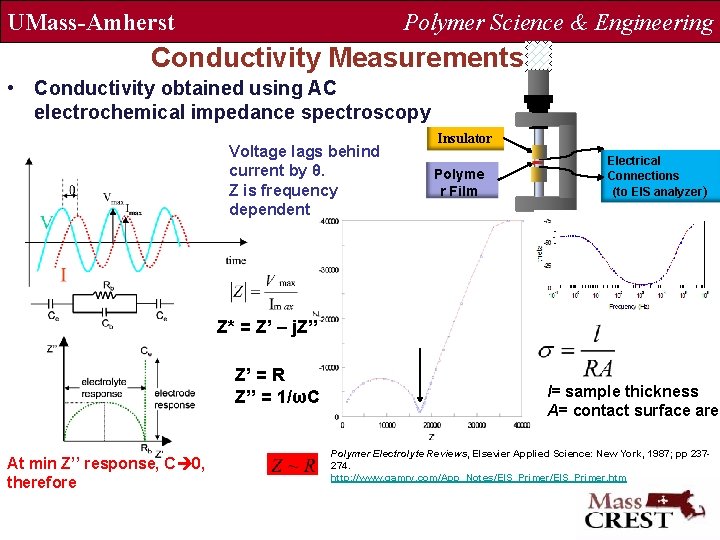
UMass-Amherst Polymer Science & Engineering Conductivity Measurements • Conductivity obtained using AC electrochemical impedance spectroscopy Voltage lags behind current by θ. Z is frequency dependent Insulator Polyme r Film Electrical Connections (to EIS analyzer) Z* = Z’ – j. Z” Z’ = R Z’’ = 1/ωC At min Z’’ response, C 0, therefore l= sample thickness A= contact surface area Polymer Electrolyte Reviews, Elsevier Applied Science: New York, 1987; pp 237274. http: //www. gamry. com/App_Notes/EIS_Primer. htm

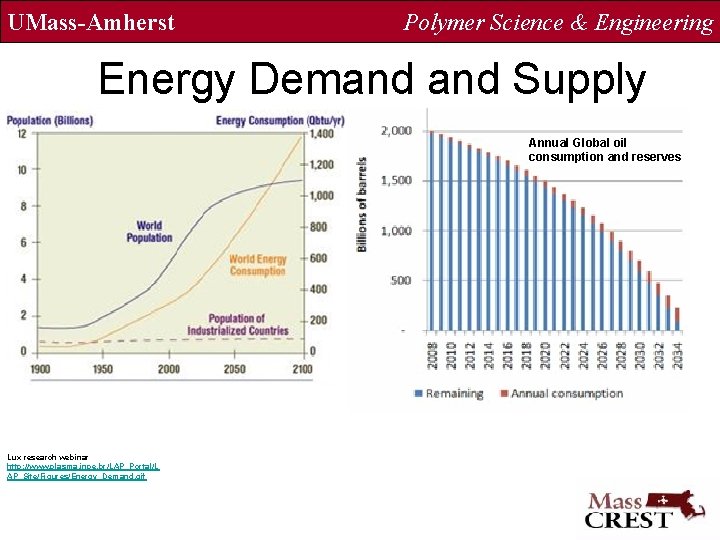
UMass-Amherst Polymer Science & Engineering Energy Demand Supply Annual Global oil consumption and reserves Lux research webinar http: //www. plasma. inpe. br/LAP_Portal/L AP_Site/Figures/Energy_Demand. gif

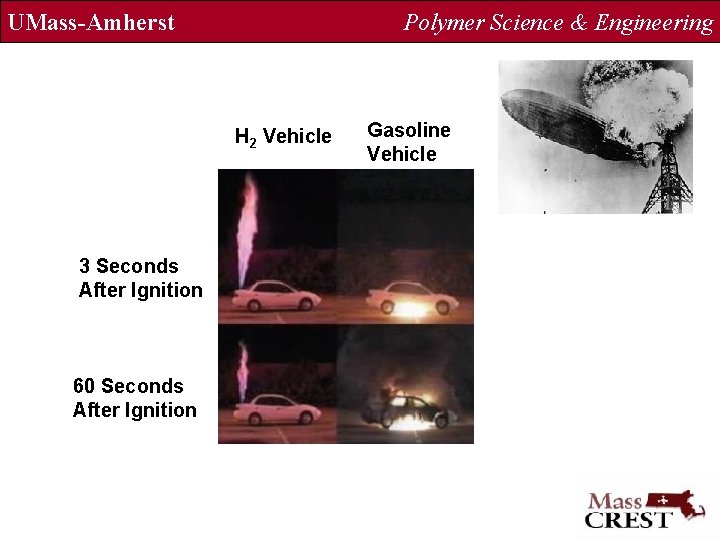
UMass-Amherst Polymer Science & Engineering H 2 Vehicle 3 Seconds After Ignition 60 Seconds After Ignition Gasoline Vehicle