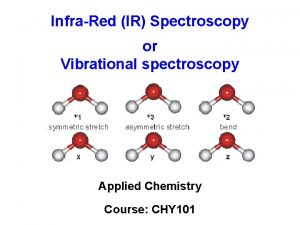
Ultraviolet and infrared spectroscopy of helical peptides and








- Slides: 19

Ultraviolet and infrared spectroscopy of helical peptides and their water complexes Jaime A. Stearns, Monia Guidi, Caroline Seaiby, Natalia Nagornova Annette Svendsen, Oleg V. Boyarkin, and Thomas R. Rizzo Laboratoire de Chimie Physique Moléculaire Ecole Polytechnique Fédérale de Lausanne 10/3/2020

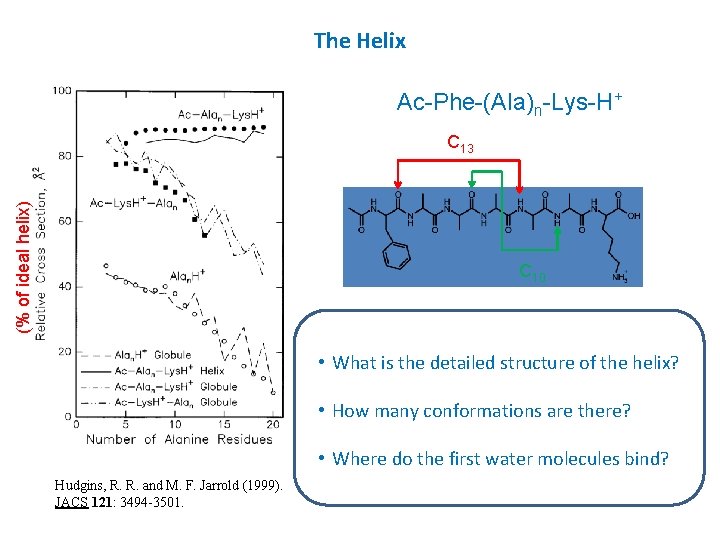
The Helix Ac-Phe-(Ala)n-Lys-H+ (% of ideal helix) C 13 C 10 • What is the detailed structure of the helix? • How many conformations are there? • Where do the first water molecules bind? Hudgins, R. R. and M. F. Jarrold (1999). JACS 121: 3494 -3501.

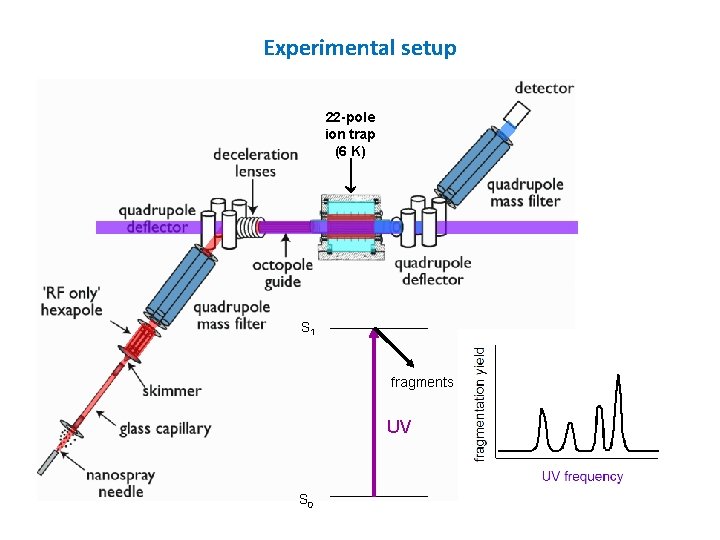
Experimental setup 22 -pole ion trap (6 K) S 1 fragments UV S 0

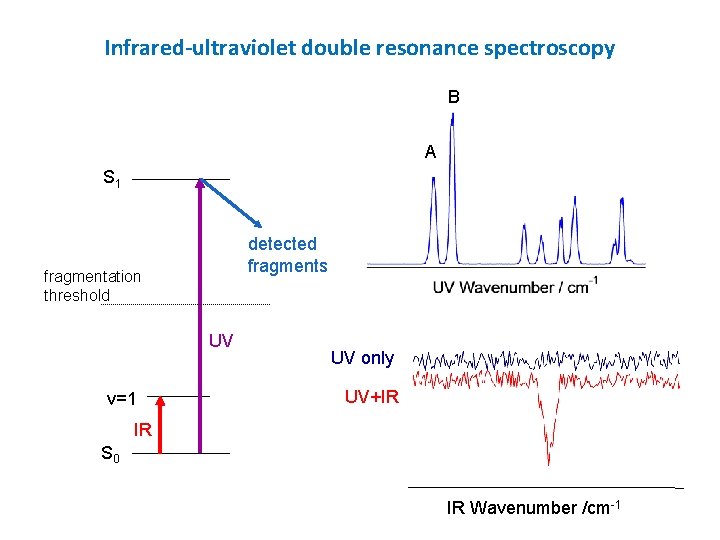
Infrared-ultraviolet double resonance spectroscopy B A S 1 detected fragments fragmentation threshold UV v=1 UV only UV+IR IR S 0 IR Wavenumber /cm-1

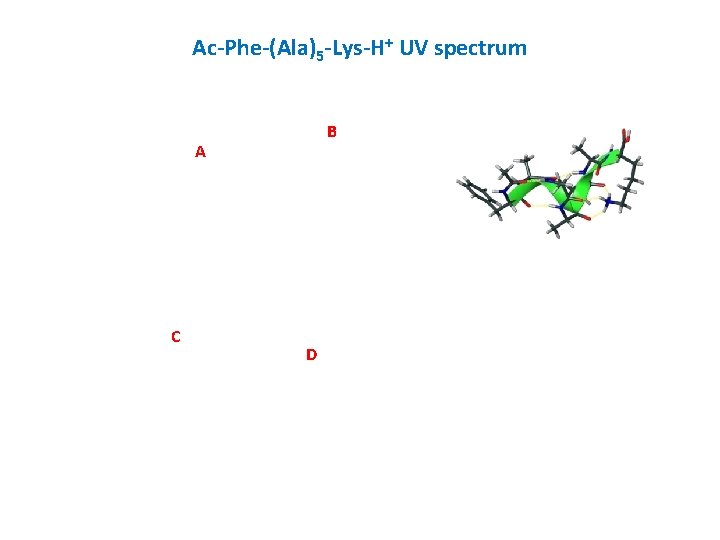
Ac-Phe-(Ala)5 -Lys-H+ UV spectrum B A C D

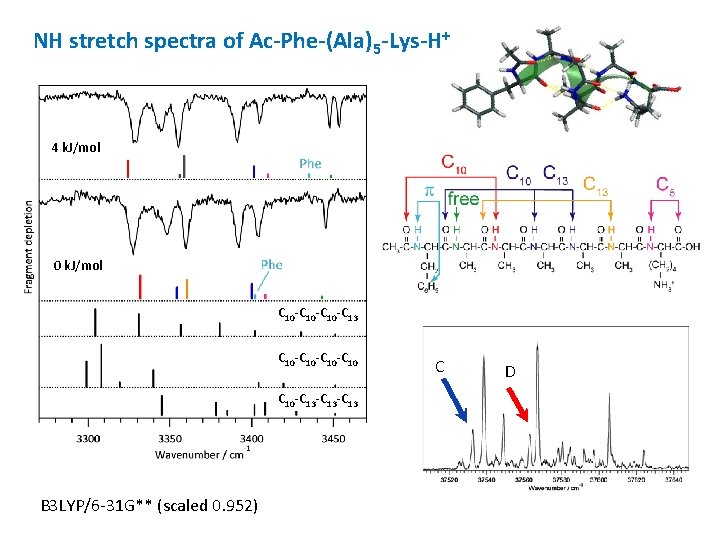
NH stretch spectra of Ac-Phe-(Ala)5 -Lys-H+ A 7 k. J/mol B 3 k. J/mol 0 k. J/mol C 10 -C 13 -C 13 C 10 -C 10 C 10 -C 13 -C 13 B 3 LYP/6 -31 G** (scaled 0. 952) A B

NH stretch spectra of Ac-Phe-(Ala)5 -Lys-H+ 4 k. J/mol 0 k. J/mol C 10 -C 13 C 10 -C 10 C 10 -C 13 -C 13 B 3 LYP/6 -31 G** (scaled 0. 952) C D

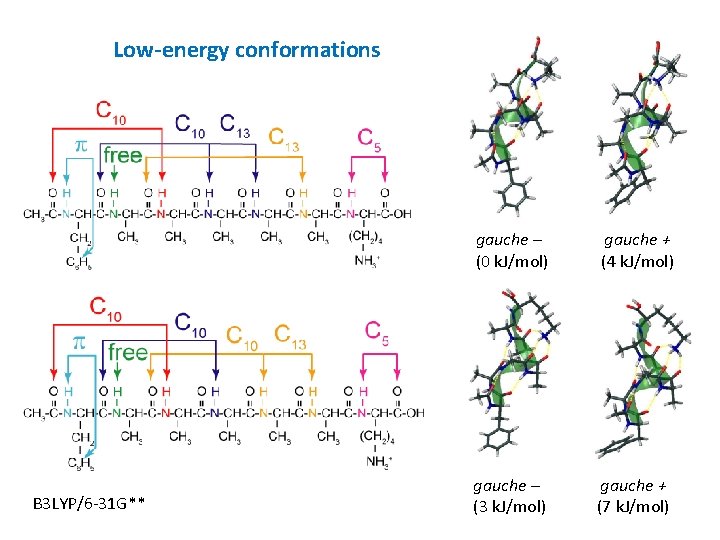
Low-energy conformations B 3 LYP/6 -31 G** gauche – (0 k. J/mol) gauche + (4 k. J/mol) gauche – (3 k. J/mol) gauche + (7 k. J/mol)

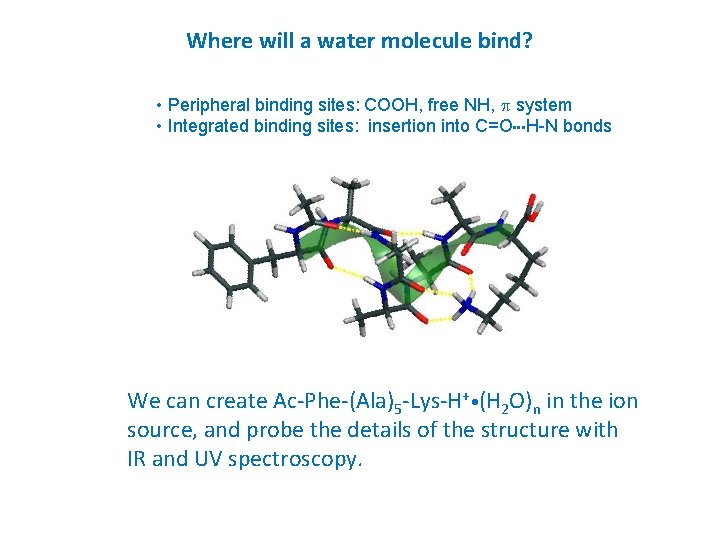
Where will a water molecule bind? • Peripheral binding sites: COOH, free NH, p system • Integrated binding sites: insertion into C=O • • • H-N bonds We can create Ac-Phe-(Ala)5 -Lys-H+ • (H 2 O)n in the ion source, and probe the details of the structure with IR and UV spectroscopy.

Creating water complexes in the ion source Ac-Phe-(Ala)5 -Lys-H+ • (H 2 O)n *impurity

UV spectra of Ac-Phe-(Ala)5 -Lys-H+ • (H 2 O)n

UV spectra of Ac-Phe-(Ala)5 -Lys-H+ • (H 2 O)n

IR spectrum of Ac-Phe-(Ala)5 -Lys-H+ • (H 2 O)1 v=1 IR monomer v=0 water complex free OH

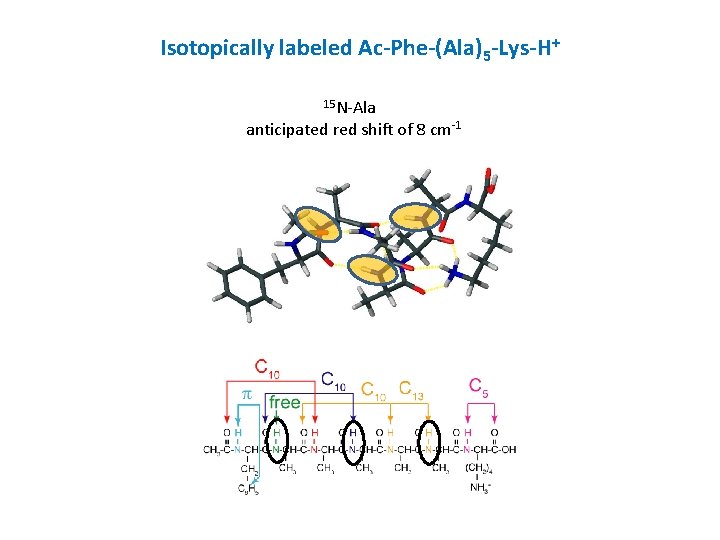
Isotopically labeled Ac-Phe-(Ala)5 -Lys-H+ 15 N-Ala anticipated red shift of 8 cm-1

Isotopically labeled Ac-Phe-(Ala)5 -Lys-H+ monomer Ala 4 Ala 6 Ala 2

Ac. FA 5 K-H+-(H 2 O)1 isotopic substitution Ala 4 Ala 6 Ala 4 Ala 2

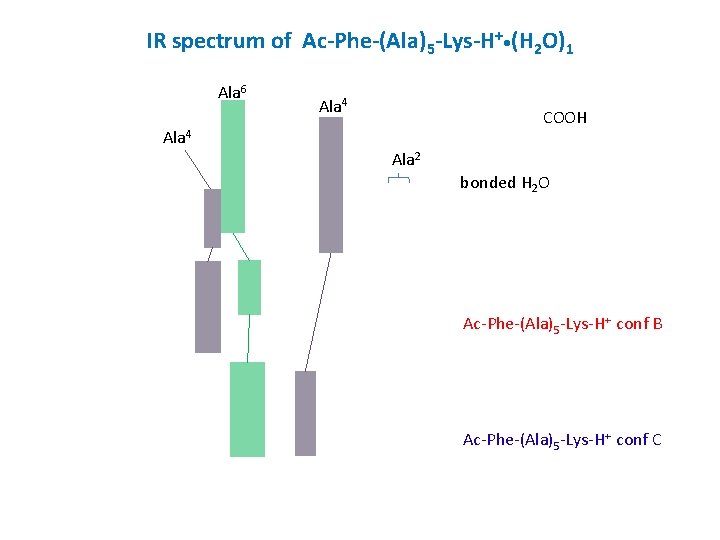
IR spectrum of Ac-Phe-(Ala)5 -Lys-H+ • (H 2 O)1 Ala 6 Ala 4 COOH Ala 4 Ala 2 bonded H 2 O Ac-Phe-(Ala)5 -Lys-H+ conf B Ac-Phe-(Ala)5 -Lys-H+ conf C

Conclusions • Ac-Phe-(Ala)5 -Lys-H+ is a helical peptide with four conformations at low temperature (10 -15 K) • We can create complexes between this peptide and more than 15 water molecules • The UV spectra indicate the first four water molecules attach away from the chromophore, likely near the charge • The fifth water molecule binds near the chromophore • The first water molecule binds in a double-donor motif • Both helical backbones are retained upon binding of the first water molecule

Acknowledgements Prof. Tom Rizzo Dr. Oleg Boyarkin Dr. Annette Svendsen Dr. Catherine Servis, Protein and Peptide Chemistry Facility Ulrich Lorenz Monia Guidi George Papadopoulos Funding: FN Caroline Seaiby Natalia Nagornova SNF
 Near infrared spectroscopy instrumentation
Near infrared spectroscopy instrumentation Nitro group ir spectrum
Nitro group ir spectrum Infrared spectroscopy theory
Infrared spectroscopy theory Infrared spectroscopy ppt
Infrared spectroscopy ppt Infrared spectroscopy
Infrared spectroscopy Amidomalonate synthesis mechanism
Amidomalonate synthesis mechanism Mobile phase in affinity chromatography
Mobile phase in affinity chromatography Cell penetrating peptides
Cell penetrating peptides Most reactions take place in a number of
Most reactions take place in a number of Bromocripine
Bromocripine Tuliskan pengertian penginderaan jauh menurut sabins
Tuliskan pengertian penginderaan jauh menurut sabins Panjang gelombang inframerah
Panjang gelombang inframerah Uv amplitude
Uv amplitude Forensic photography management
Forensic photography management Characteristics of ultraviolet rays
Characteristics of ultraviolet rays Euv lithography ppt
Euv lithography ppt Ultraviolet catastrophe
Ultraviolet catastrophe Lewis structure h2co
Lewis structure h2co Crus of helix
Crus of helix Hawley appliance with expansion screw
Hawley appliance with expansion screw