TRICHLOROETHYLENE TCE MW 131 Cl H C C






















- Slides: 32

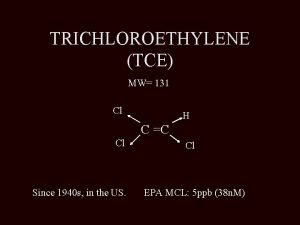
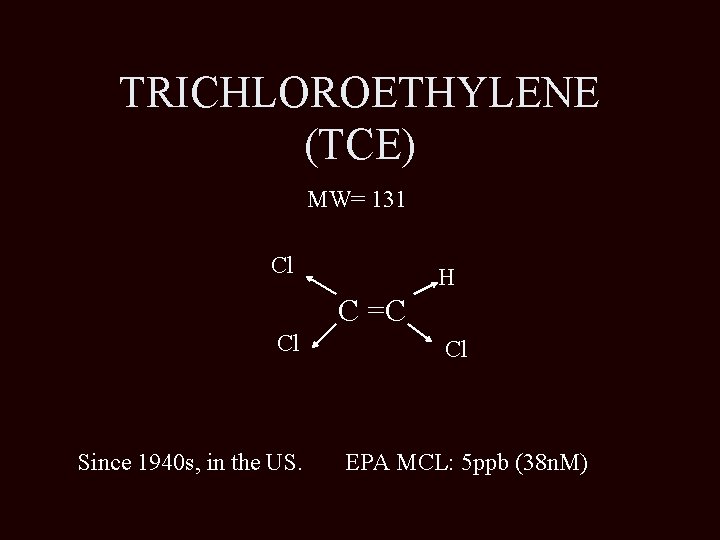
TRICHLOROETHYLENE (TCE) MW= 131 Cl H C =C Cl Since 1940 s, in the US. Cl EPA MCL: 5 ppb (38 n. M)

TCE • Industrial degreasing agent • Solvent used in dry cleaning solutions, paint removers, cosmetics, adhesives, household cleaners, and spot removers. • Anesthetic • Chlorination product (TCAA, DCA)

TCE : WHERE? • Air : Urban 3 times > than rural. • Water: rain, surface water, groundwater, drinking water, and sea water. • Marine sediments, marine invertebrates, marine mammals, foods, mother’s milk, human urine and blood. • Foods: butter and margarine, cheese, processed food, cereals.

TCE IN GROUNDWATER • 93% of the public water system obtained from groundwater • Between 9 and 34% of drinking water supply sources tested in the US have some TCE contamination. TCAA and DCA are products of chlorination. • MCL violations are rare for any extended period. • Private wells?

TCE TARGET OF EXPOSURE • Lung • Gastrointestinal tract • Skin

TCE AND CANCER • IN ANIMALS: • Liver, kidney, lung tumors and lymphomas in rats and mice.

TCE AND CANCER • IN HUMANS: • Kidney and liver cancers, lymphomas (Non. Hodgkin’s and Hodgkin’s disease). • Pancreatic cancer, multiple myeloma, prostate and skin cancer, leukemia.

CONCLUSION • TCE IS A PROBABLE CARCINOGEN TO HUMAN BASED ON LIMITED HUMAN EVIDENCE AND SUFFICIENT ANIMAL EVIDENCE (International Agency for Research on Cancer, 1995)

NONCANCER EFFECTS OF TCE • CNS : Dizziness, headache, sleepiness, nausea, confusion, blurred vision, weakness (acute exposure) • Reproductive/Developmental: Miscarriage, cardiac defects, eye malformations, neural tube and oral cleft defects, abnormal sperm morphology(mice), hearing and speech impairment and increase in urinary tract disorders (children 0 -9 years of age).

Congenital Heart Disease • Affects almost 1% of newborns and may account for 2 -10% of stillbirths and spontaneous abortions • Has a significant environmental component as only about 20% of defects have a clear genetic cause • Can be caused by retinoic acid(+++), TCE (3), aspirin (2), fetal alcohol (? ), cocaine (? ) • Higher in Yuma and transborder areas (environmental? )

TCE is a Cardiac Teratogen • Epidemiologic Studies • Animal Studies (chick, rat, in utero and drinking water exposure) • In vitro Experiments (Collagen gel assay)

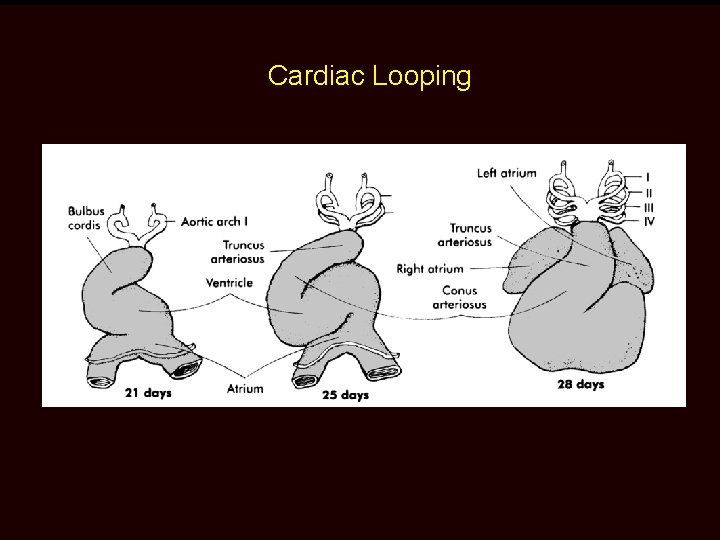
Cardiac Looping

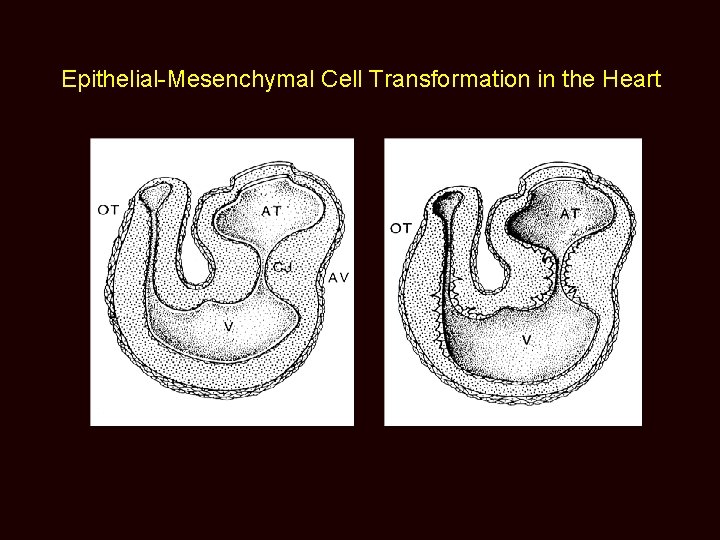
Epithelial-Mesenchymal Cell Transformation in the Heart


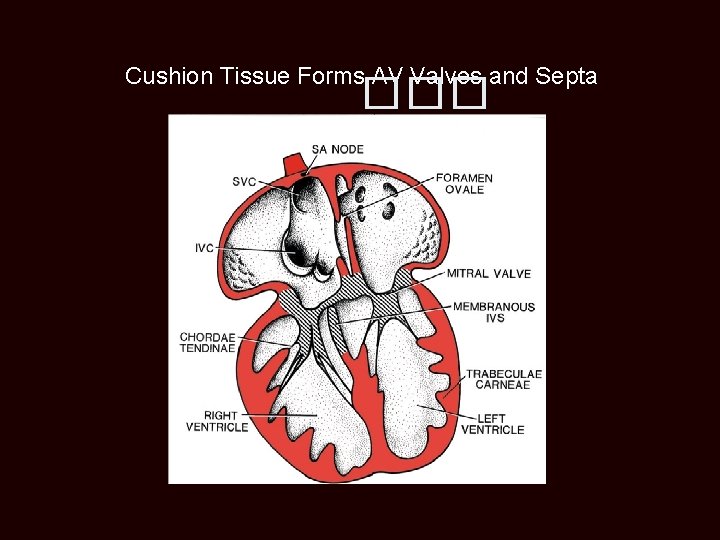
Cushion Tissue Forms AV Valves and Septa ���

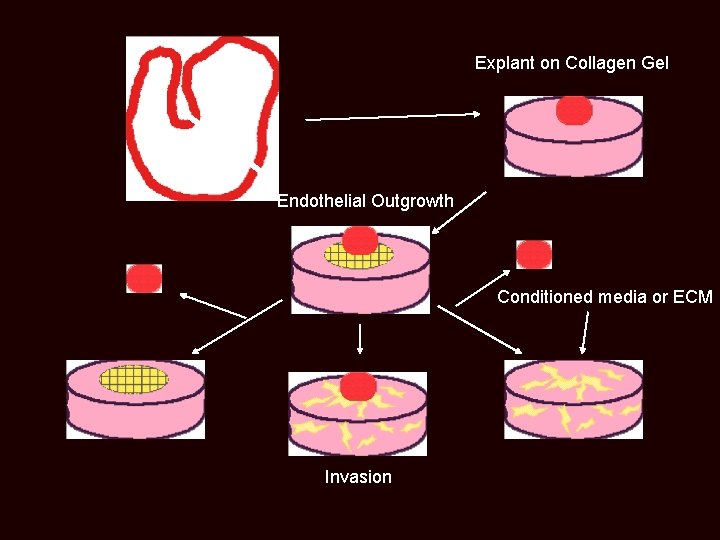
Explant on Collagen Gel Endothelial Outgrowth Conditioned media or ECM Invasion

How do you find genes with altered expression?

Hypothesis Molecules with altered expression in the rat embryo, as a consequence of maternal exposure to TCE (or As), can be used to identify actual effectors of birth defects. Alternatively, they might prove useful as specific biomarkers of environmental exposure.

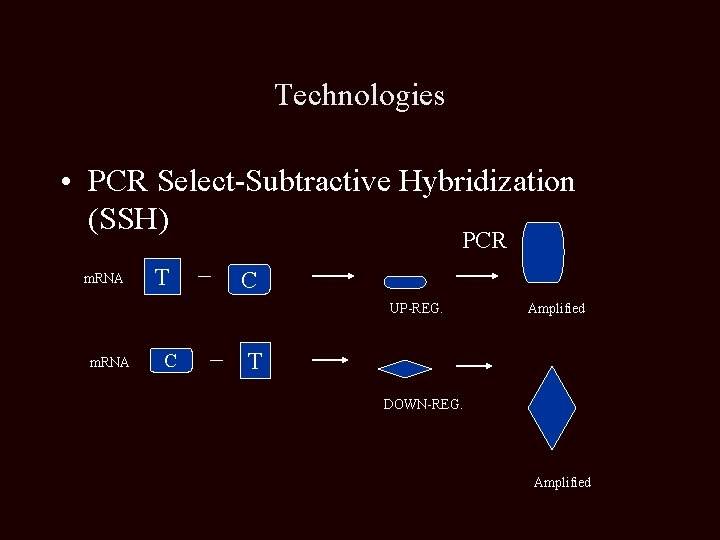
Technologies • PCR Select-Subtractive Hybridization (SSH) PCR m. RNA T C UP-REG. m. RNA C Amplified T DOWN-REG. Amplified

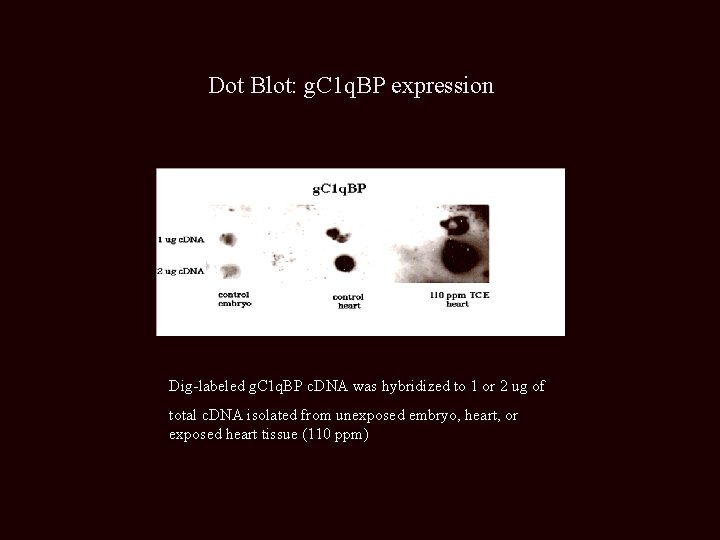
Dot Blot: g. C 1 q. BP expression Dig-labeled g. C 1 q. BP c. DNA was hybridized to 1 or 2 ug of total c. DNA isolated from unexposed embryo, heart, or exposed heart tissue (110 ppm)

p 137 • GPI-linked protein identified in Caco-2 cells (Ellis et al. , 1992), and in several tissues. Possible function as second messenger. • Very conserved among species (human, rat, mouse, chicken)




The SERCA Family • Sarco/endoplasmic reticulum Ca+2 ATPases, mediating the uptake of Ca+2 into intracellular stores. • Encoded by three genes in higher vertebrates: SERCA 1, 2, 3.

TCE down-regulated SERCA 2 • The differentially expressed c. DNA isolated from cardiac embryonic tissue is 300 bp. • Blast analysis indicates 94 -95% homology to the SERCA 2 gene, in the 3’ untranslated region common to both SERCA 2 a and b transcripts.

SERCA 2 TRANSGENIC MICE • Serca 2 a Null heterozygous: Ca++ uptake into the SR. No outward phenotype (Periasamy et al. 1999) • Serca 2 a Overexpressed: Calcium transients, myocardial contractility and relaxation (He et al. , 1997) • Serca 2 b Overexpressed: SR calcium transport function and cardiac contractility (Greene et al. , 2000)

Future Studies • Utilize transgenic mice (over-expressing or null heterozygous for SERCA 2) to test their altered sensitivity to TCE in vitro.


SUMMARY • Several potential markers of TCE exposure in the developing heart have been isolated. • Characterization of function and expression is in progress. • Tools for identification of proximal effectors of TCE induced heart defects.

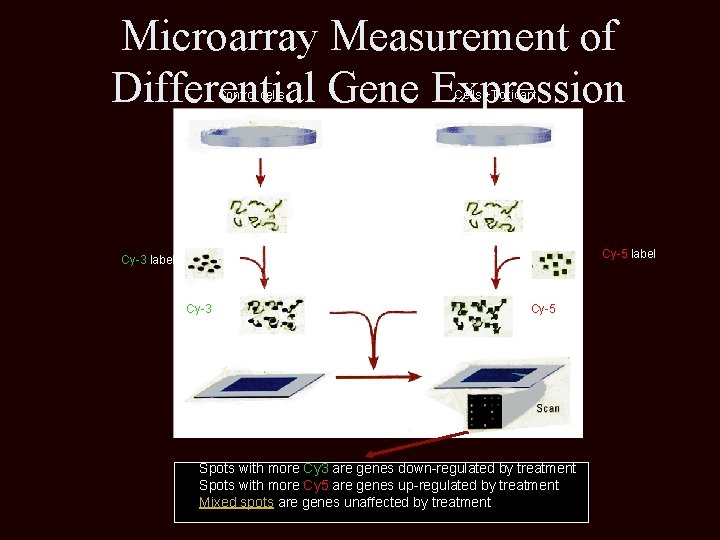
Microarray Measurement of Differential Gene Expression Control cells Cells +Toxicant RNA isolation Reverse transcription Cy-3 labeled c. DNA Cy-5 labeled c. DNA Mix c. DNAs and apply to array. Hybridize under coverslip. Spots with more Cy 3 are genes down-regulated by treatment Spots with more Cy 5 are genes up-regulated by treatment Mixed spots are genes unaffected by treatment

Future Directions • Elucidate the molecular pathways used by TCE to disrupt normal development. • Use the most sensitive bio markers for translation into prevention and/or intervention measures on affected populations.
 Hematoma epidural
Hematoma epidural Tce
Tce Tce easement
Tce easement Tce theory
Tce theory Tce mw
Tce mw Tucson tce contamination map
Tucson tce contamination map Tce
Tce Hmn-131
Hmn-131 Kristin spent $131 on shirts answers
Kristin spent $131 on shirts answers Cmsc 131
Cmsc 131 Hpd 113 uitm
Hpd 113 uitm Buderus schulung
Buderus schulung Quantas meias vidas deve transcorrer para que 93 75
Quantas meias vidas deve transcorrer para que 93 75 Cse 132
Cse 132 Half life
Half life Could 131g of xenon gas in a vessel
Could 131g of xenon gas in a vessel 131 muhasebe kodu
131 muhasebe kodu Cmsc 131
Cmsc 131 Psalm 131 vers 4
Psalm 131 vers 4 Phy 131 asu
Phy 131 asu Cogsci 131
Cogsci 131 Medical plaza miller 131 miller street
Medical plaza miller 131 miller street The ascent humble
The ascent humble Mis 131
Mis 131 Salmo 131 2
Salmo 131 2 Phy 131 past papers
Phy 131 past papers Violetta 131
Violetta 131 Pp-131
Pp-131 Cmpe 131
Cmpe 131 Cmsc 131
Cmsc 131 Cse 131
Cse 131 Olivier moindrot
Olivier moindrot Mis 131
Mis 131