Thermodynamics of oxygen defective Ti O 2 x

- Slides: 16

Thermodynamics of oxygen defective Ti. O 2 -x : The Magneli phases. Leandro Liborio Giuseppe Mallia Nicholas Harrison Computational Materials Science Group

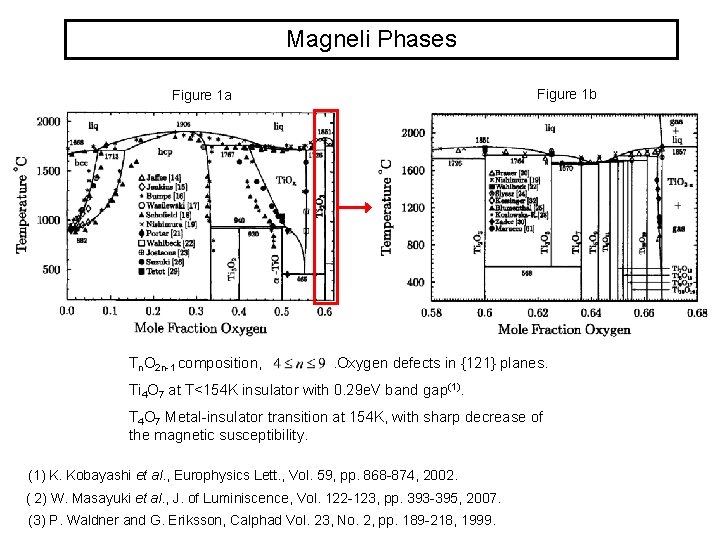
Magneli Phases Figure 1 b Figure 1 a Tn. O 2 n-1 composition, . Oxygen defects in {121} planes. Ti 4 O 7 at T<154 K insulator with 0. 29 e. V band gap(1). T 4 O 7 Metal-insulator transition at 154 K, with sharp decrease of the magnetic susceptibility. (1) K. Kobayashi et al. , Europhysics Lett. , Vol. 59, pp. 868 -874, 2002. ( 2) W. Masayuki et al. , J. of Luminiscence, Vol. 122 -123, pp. 393 -395, 2007. (3) P. Waldner and G. Eriksson, Calphad Vol. 23, No. 2, pp. 189 -218, 1999.

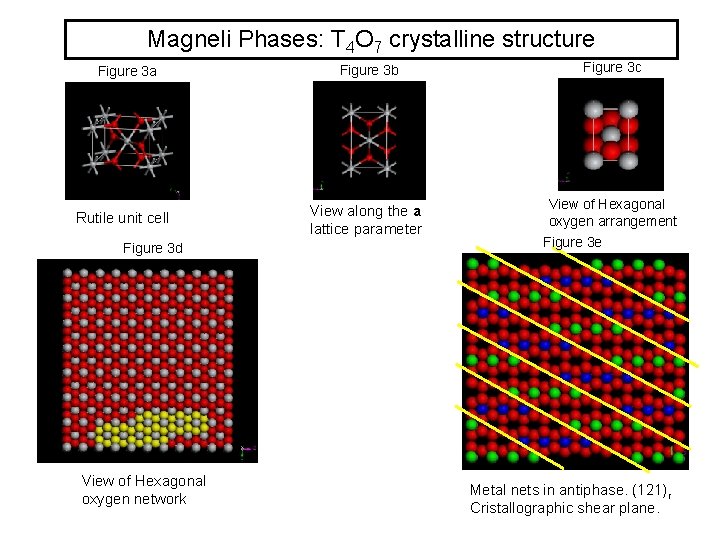
Magneli Phases: T 4 O 7 crystalline structure Figure 3 a Rutile unit cell Figure 3 d View of Hexagonal oxygen network Figure 3 b Figure 3 c View along the a lattice parameter View of Hexagonal oxygen arrangement Figure 3 e Metal nets in antiphase. (121)r Cristallographic shear plane.

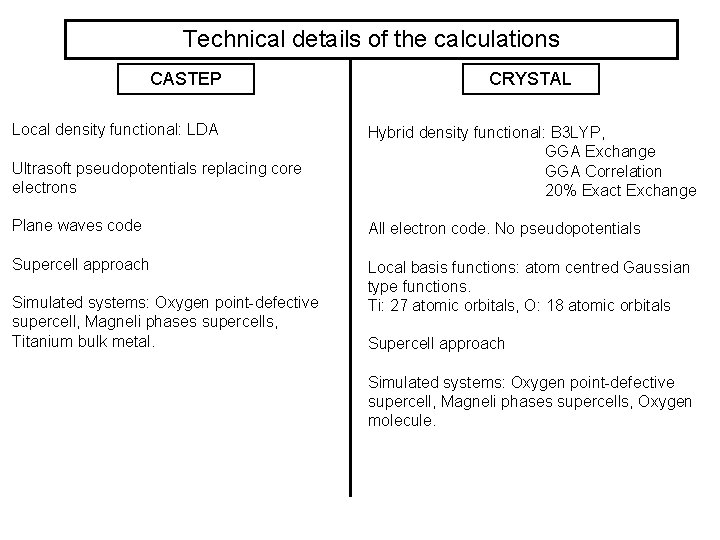
Technical details of the calculations CASTEP Local density functional: LDA CRYSTAL Ultrasoft pseudopotentials replacing core electrons Hybrid density functional: B 3 LYP, GGA Exchange GGA Correlation 20% Exact Exchange Plane waves code All electron code. No pseudopotentials Supercell approach Local basis functions: atom centred Gaussian type functions. Ti: 27 atomic orbitals, O: 18 atomic orbitals Simulated systems: Oxygen point-defective supercell, Magneli phases supercells, Titanium bulk metal. Supercell approach Simulated systems: Oxygen point-defective supercell, Magneli phases supercells, Oxygen molecule.

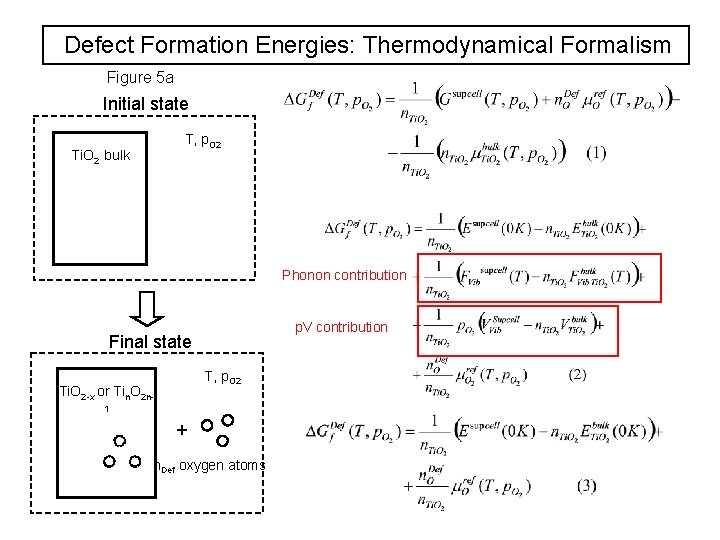
Defect Formation Energies: Thermodynamical Formalism Figure 5 a Initial state T, p. O 2 Ti. O 2 bulk Phonon contribution p. V contribution Final state T, p. O 2 Ti. O 2 -x or Tin. O 2 n 1 + n. Def oxygen atoms

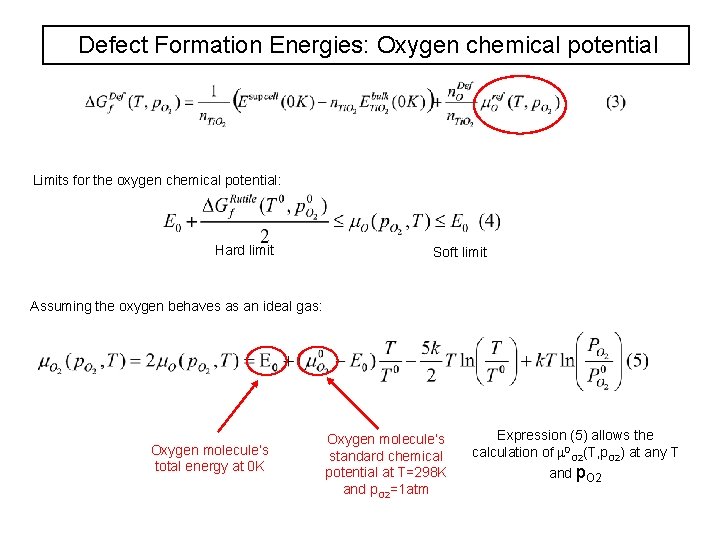
Defect Formation Energies: Oxygen chemical potential Limits for the oxygen chemical potential: Hard limit Soft limit Assuming the oxygen behaves as an ideal gas: Oxygen molecule’s total energy at 0 K Oxygen molecule’s standard chemical potential at T=298 K and p. O 2=1 atm Expression (5) allows the calculation of 0 O 2(T, p. O 2) at any T and p. O 2

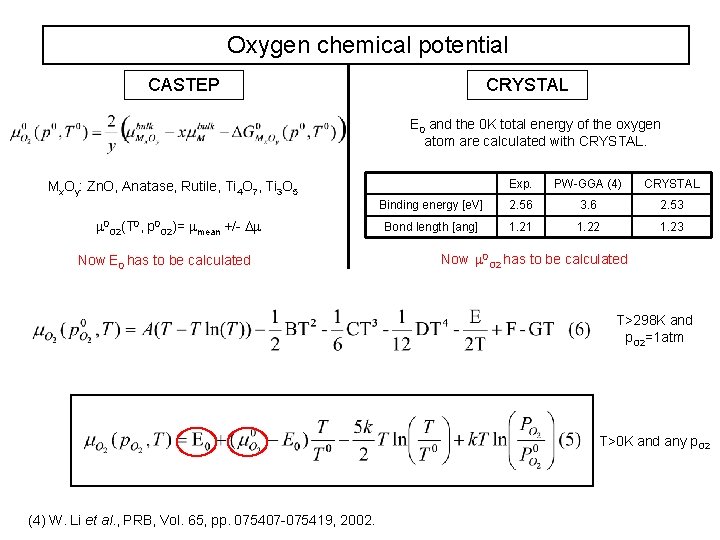
Oxygen chemical potential CASTEP CRYSTAL E 0 and the 0 K total energy of the oxygen atom are calculated with CRYSTAL. Exp. PW-GGA (4) CRYSTAL Binding energy [e. V] 2. 56 3. 6 2. 53 Bond length [ang] 1. 21 1. 22 1. 23 Mx. Oy: Zn. O, Anatase, Rutile, Ti 4 O 7, Ti 3 O 5 0 O 2(T 0, p 0 O 2)= mean +/- Now E 0 has to be calculated Now 0 O 2 has to be calculated T>298 K and p. O 2=1 atm T>0 K and any p. O 2 (4) W. Li et al. , PRB, Vol. 65, pp. 075407 -075419, 2002.

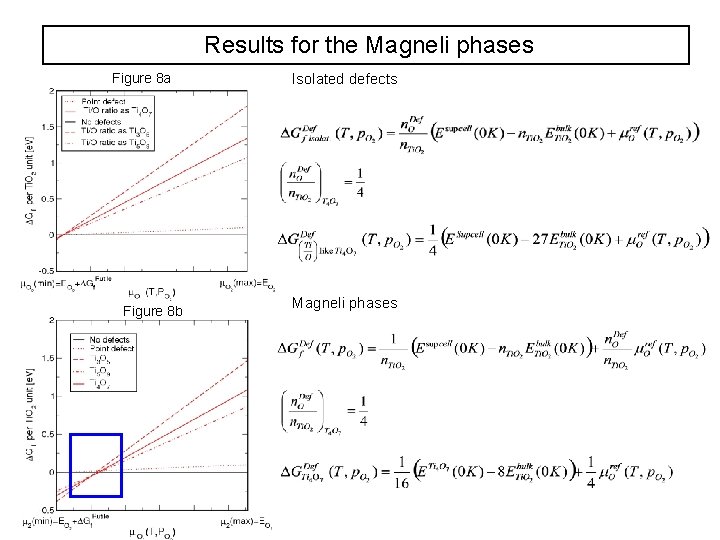
Results for the Magneli phases Figure 8 a Figure 8 b Isolated defects Magneli phases

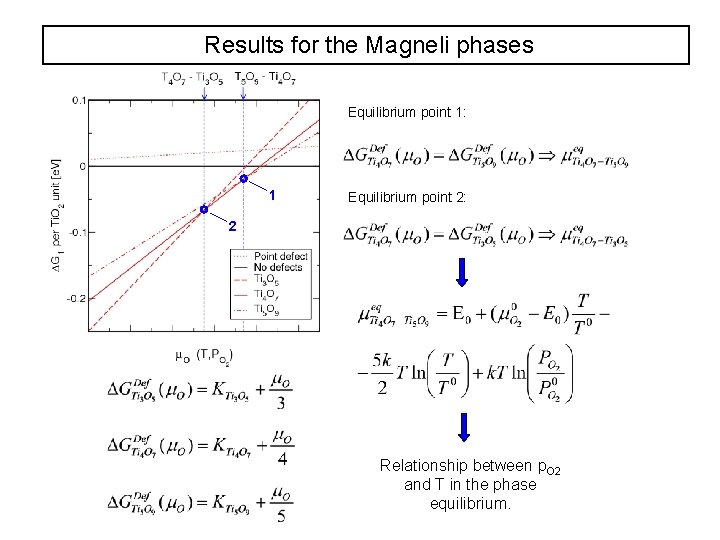
Results for the Magneli phases Equilibrium point 1: 1 Equilibrium point 2: 2 Relationship between p. O 2 and T in the phase equilibrium.

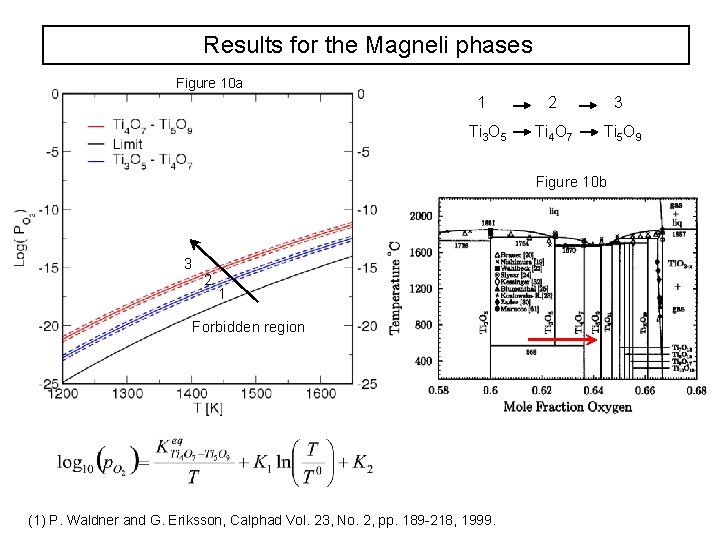
Results for the Magneli phases Figure 10 a 1 Ti 3 O 5 2 3 Ti 4 O 7 Ti 5 O 9 Figure 10 b 3 2 1 Forbidden region (1) P. Waldner and G. Eriksson, Calphad Vol. 23, No. 2, pp. 189 -218, 1999.

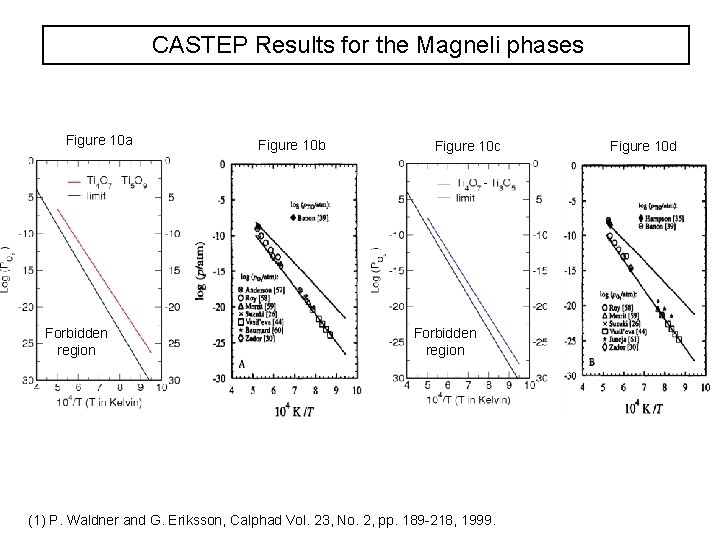
CASTEP Results for the Magneli phases Figure 10 a Forbidden region Figure 10 b Figure 10 c Forbidden region (1) P. Waldner and G. Eriksson, Calphad Vol. 23, No. 2, pp. 189 -218, 1999. Figure 10 d

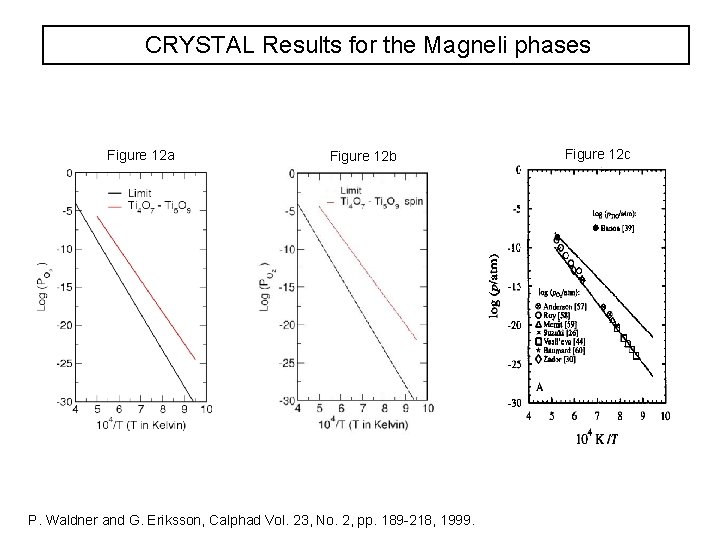
CRYSTAL Results for the Magneli phases Figure 12 a Figure 12 b P. Waldner and G. Eriksson, Calphad Vol. 23, No. 2, pp. 189 -218, 1999. Figure 12 c

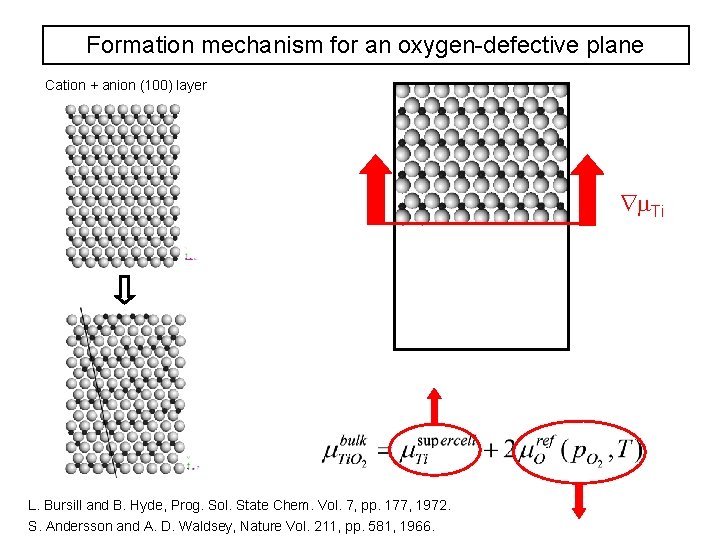
Formation mechanism for an oxygen-defective plane Cation + anion (100) layer Ti L. Bursill and B. Hyde, Prog. Sol. State Chem. Vol. 7, pp. 177, 1972. S. Andersson and A. D. Waldsey, Nature Vol. 211, pp. 581, 1966.

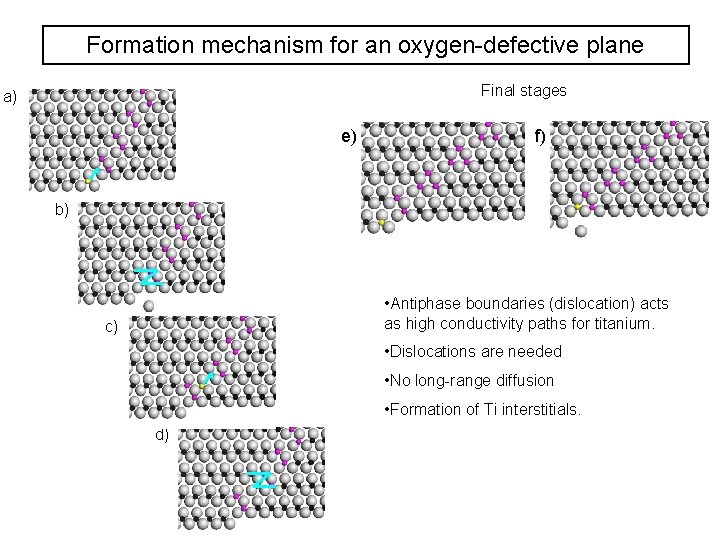
Formation mechanism for an oxygen-defective plane Final stages a) e) f) b) • Antiphase boundaries (dislocation) acts as high conductivity paths for titanium. c) • Dislocations are needed • No long-range diffusion • Formation of Ti interstitials. d)

Conclusions • The thermodynamics of rutile’s higher oxides has been investigated by first principles calculations. • First principles thermodynamics reproduce the experimental observations reasonably well. • Spin does not affect thermodynamics. • At a high concentration of oxygen defects and low oxygen chemical potential, oxygen defects prefer to form Magneli phases. • But, at low concentration of oxygen defects and low oxygen chemical potential, titanium interstitials proved to be the stable point defects. • These results support the mechanism proposed by Andersson and Waldsey for the production the crystalline shear planes in rutile.

Acknowledgements • Prof. Nic Harrison • Dr. Giuseppe Mallia • Dr. Barbara Montanari • Dr Keith Refson
 Family limited partnership
Family limited partnership Proximate norm of morality examples
Proximate norm of morality examples A package contains 12 resistors 3 of which are defective
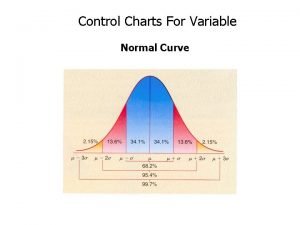
A package contains 12 resistors 3 of which are defective Normal distribution chart
Normal distribution chart Divide should ideas prevail
Divide should ideas prevail Intentionally defective grantor trust diagram
Intentionally defective grantor trust diagram Collective inferential error
Collective inferential error Regular superlatives
Regular superlatives Intentionally defective grantor trust diagram
Intentionally defective grantor trust diagram Tumor twins 7 habits
Tumor twins 7 habits Defective metering valve
Defective metering valve Unit 5 quiz 1 power assist systems
Unit 5 quiz 1 power assist systems Defective mand example
Defective mand example Genuine agreement
Genuine agreement Moral sensism example
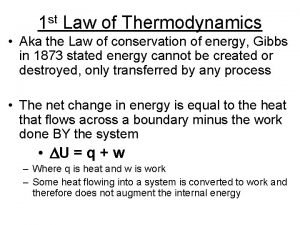
Moral sensism example Thermodynamics is concerned with
Thermodynamics is concerned with Dq=cdt
Dq=cdt