THE USE OF ACELLULAR DERMIS IN LEG ULCER






- Slides: 22

THE USE OF “ACELLULAR DERMIS” IN LEG ULCER MANAGEMENT Nicholas Greaves 14. 05. 12


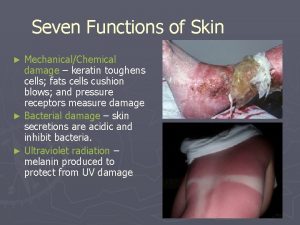
THE SIZE OF THE PROBLEM Prevalence – 1: 100 in the elderly. Increasing incidence secondary to aging population and increasingly prevalent risk factors such as diabetes, obesity and atherosclerosis. Cost: � Venous ulcers - £ 400 million/annum � Diabetic ulcers - £ 252 million/annum � Pressure ulcers - £ 300 million/annum Cost secondary to dressings, staffing and hospital admissions.

CURRENT MANAGEMENT Dependent on aetiology: � Arterial - medical therapy, angioplasty, stenting or bypass surgery. � Neuropathic/Pressure Good diabetic control Regular podiatry and foot care Pressure relieving methods � Venous - compression bandaging Success ~60% healed in 26 weeks. Labour intensive, expensive and prone to recurrence. 30 -50% of district nurse workload.

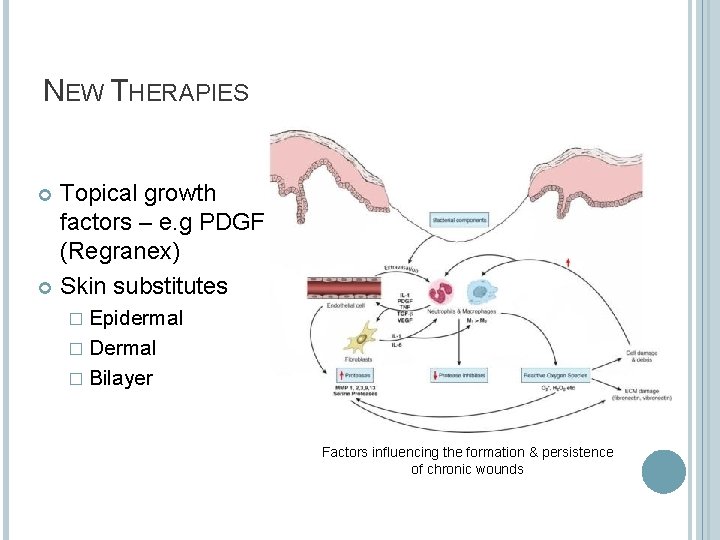
NEW THERAPIES Topical growth factors – e. g PDGF (Regranex) Skin substitutes � Epidermal � Dermal � Bilayer Factors influencing the formation & persistence of chronic wounds

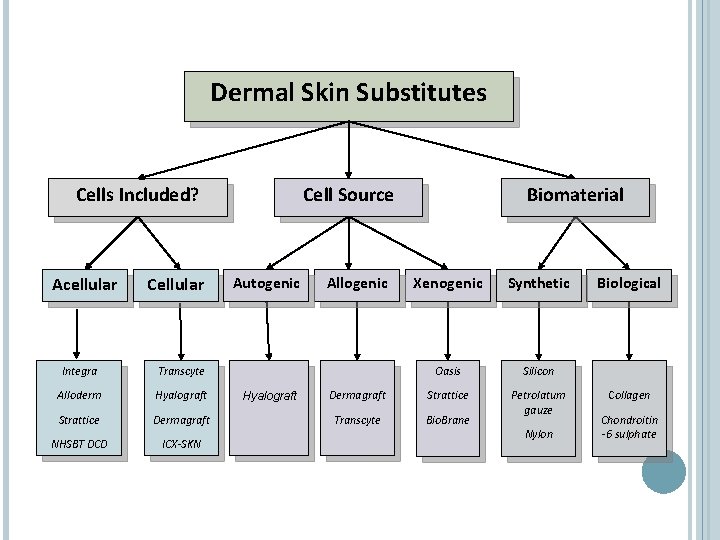
Dermal Skin Substitutes Cells Included? Acellular Cellular Integra Transcyte Alloderm Hyalograft Strattice Dermagraft NHSBT DCD ICX-SKN Cell Source Autogenic Hyalograft Allogenic Biomaterial Xenogenic Synthetic Oasis Silicon Dermagraft Strattice Transcyte Bio. Brane Petrolatum gauze Nylon Biological Collagen Chondroitin -6 sulphate

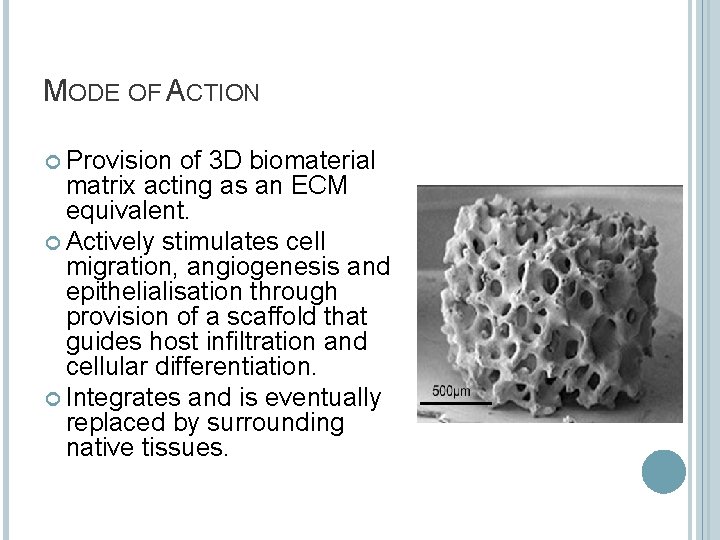
MODE OF ACTION Provision of 3 D biomaterial matrix acting as an ECM equivalent. Actively stimulates cell migration, angiogenesis and epithelialisation through provision of a scaffold that guides host infiltration and cellular differentiation. Integrates and is eventually replaced by surrounding native tissues.

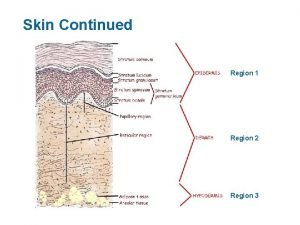
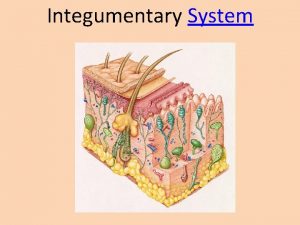
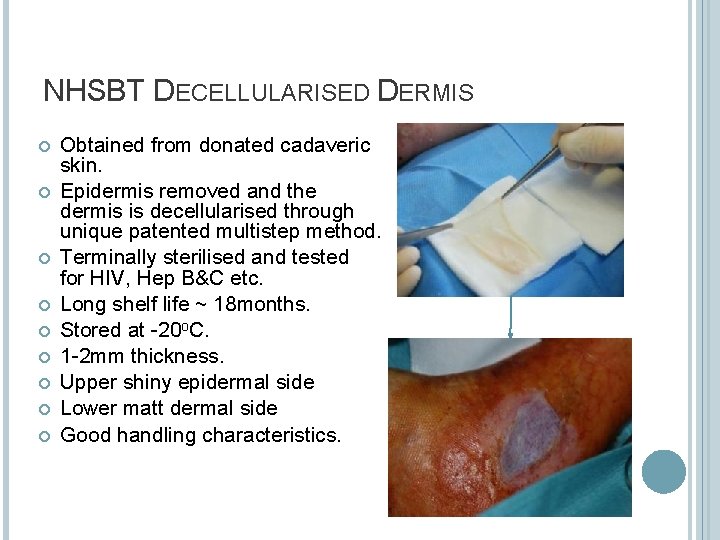
NHSBT DECELLULARISED DERMIS Obtained from donated cadaveric skin. Epidermis removed and the dermis is decellularised through unique patented multistep method. Terminally sterilised and tested for HIV, Hep B&C etc. Long shelf life ~ 18 months. Stored at -20 o. C. 1 -2 mm thickness. Upper shiny epidermal side Lower matt dermal side Good handling characteristics.

THE USE OF ACELLULAR DERMIS IN THE TREATMENT OF CHRONIC WOUNDS Pilot study of 20 patients � REC approved � Recruitment sites – South Manchester, Stockport, Trafford, Salford, Central Manchester Inclusion criteria Exclusion criteria Age > 16 BMI > 45 Competent to consent Warfarin Ulcer of lower limb Steroids Venous ulcer Rheumatoid arthritis Arterial ulcer with ABPI > 0. 7 Severe heart failure Neuropathic ulcer in nonweightbearing area Chronic kidney or liver disease >2 cm diameter Cancer in the last 5 years Pregnancy Current or ex-IVDU

STUDY DESIGN Patient deemed suitable by clinical care team to take part in the study Patient provided with information pack Patient has pre-study screening appointment Patient fits inclusion/exclusion criteria and continues to express an interest to participate, history and examination performed Duplex scan completed on lower limbs & wound swabbed Written informed consent obtained

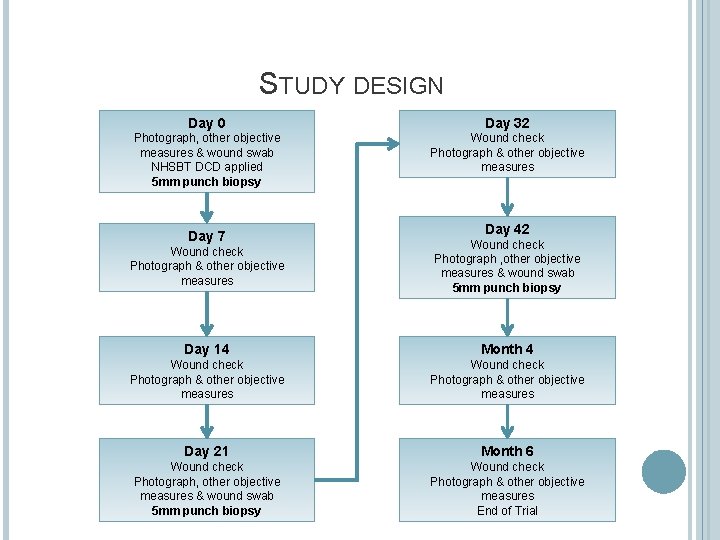
STUDY DESIGN Day 0 Day 32 Photograph, other objective measures & wound swab NHSBT DCD applied 5 mm punch biopsy Wound check Photograph & other objective measures Day 7 Day 42 Wound check Photograph & other objective measures Wound check Photograph , other objective measures & wound swab 5 mm punch biopsy Day 14 Month 4 Wound check Photograph & other objective measures Day 21 Month 6 Wound check Photograph, other objective measures & wound swab 5 mm punch biopsy Wound check Photograph & other objective measures End of Trial

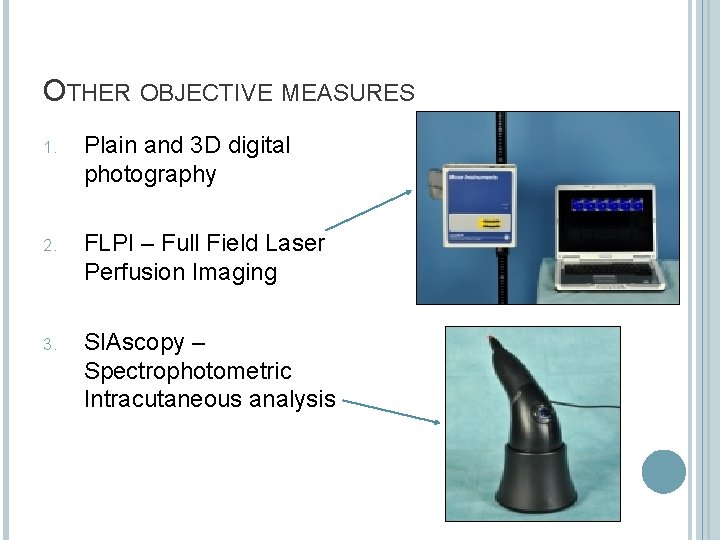
OTHER OBJECTIVE MEASURES 1. Plain and 3 D digital photography 2. FLPI – Full Field Laser Perfusion Imaging 3. SIAscopy – Spectrophotometric Intracutaneous analysis

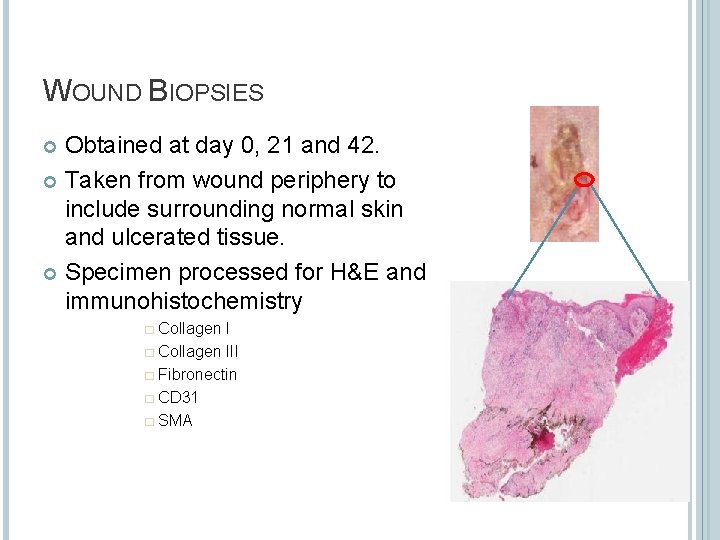
WOUND BIOPSIES Obtained at day 0, 21 and 42. Taken from wound periphery to include surrounding normal skin and ulcerated tissue. Specimen processed for H&E and immunohistochemistry � Collagen III � Fibronectin � CD 31 � SMA

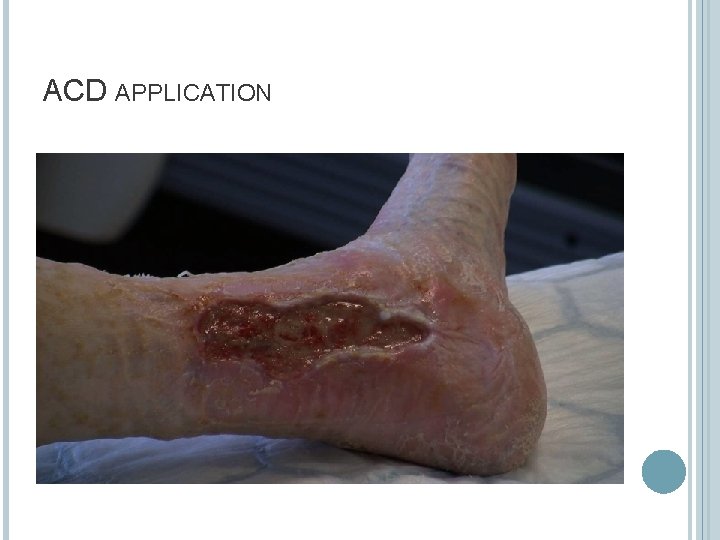
ACD APPLICATION

ADVANTAGES No hospital admission No anaesthetic Minimal analgesia required One stage application takes 15 -20 minutes. Can be used “off the shelf” and tailor fit to any wound. No split thickness skin graft with inherent second wound site.

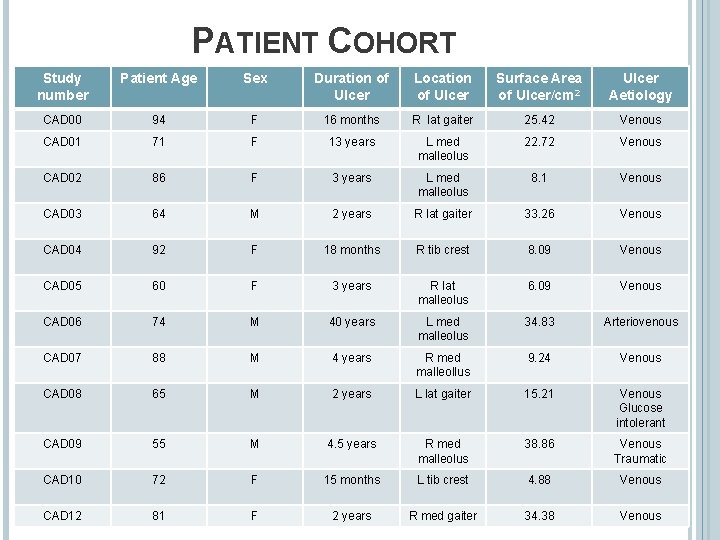
PATIENT COHORT Study number Patient Age Sex Duration of Ulcer Location of Ulcer Surface Area of Ulcer/cm 2 Ulcer Aetiology CAD 00 94 F 16 months R lat gaiter 25. 42 Venous CAD 01 71 F 13 years L med malleolus 22. 72 Venous CAD 02 86 F 3 years L med malleolus 8. 1 Venous CAD 03 64 M 2 years R lat gaiter 33. 26 Venous CAD 04 92 F 18 months R tib crest 8. 09 Venous CAD 05 60 F 3 years R lat malleolus 6. 09 Venous CAD 06 74 M 40 years L med malleolus 34. 83 Arteriovenous CAD 07 88 M 4 years R med malleollus 9. 24 Venous CAD 08 65 M 2 years L lat gaiter 15. 21 Venous Glucose intolerant CAD 09 55 M 4. 5 years R med malleolus 38. 86 Venous Traumatic CAD 10 72 F 15 months L tib crest 4. 88 Venous CAD 12 81 F 2 years R med gaiter 34. 38 Venous

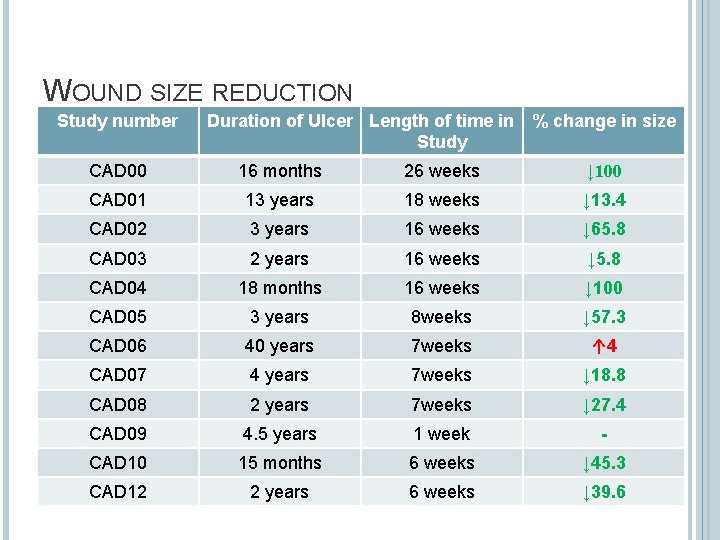
WOUND SIZE REDUCTION Study number Duration of Ulcer Length of time in % change in size Study CAD 00 16 months 26 weeks ↓ 100 CAD 01 13 years 18 weeks ↓ 13. 4 CAD 02 3 years 16 weeks ↓ 65. 8 CAD 03 2 years 16 weeks ↓ 5. 8 CAD 04 18 months 16 weeks ↓ 100 CAD 05 3 years 8 weeks ↓ 57. 3 CAD 06 40 years 7 weeks ↑ 4 CAD 07 4 years 7 weeks ↓ 18. 8 CAD 08 2 years 7 weeks ↓ 27. 4 CAD 09 4. 5 years 1 week - CAD 10 15 months 6 weeks ↓ 45. 3 CAD 12 2 years 6 weeks ↓ 39. 6

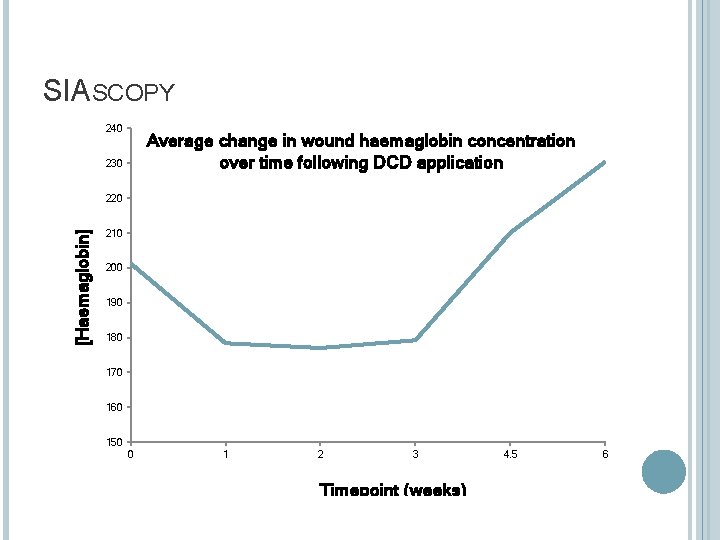
SIASCOPY 240 Average change in wound haemaglobin concentration over time following DCD application 230 [Haemaglobin] 220 210 200 190 180 170 160 150 0 1 2 3 Timepoint (weeks) 4. 5 6

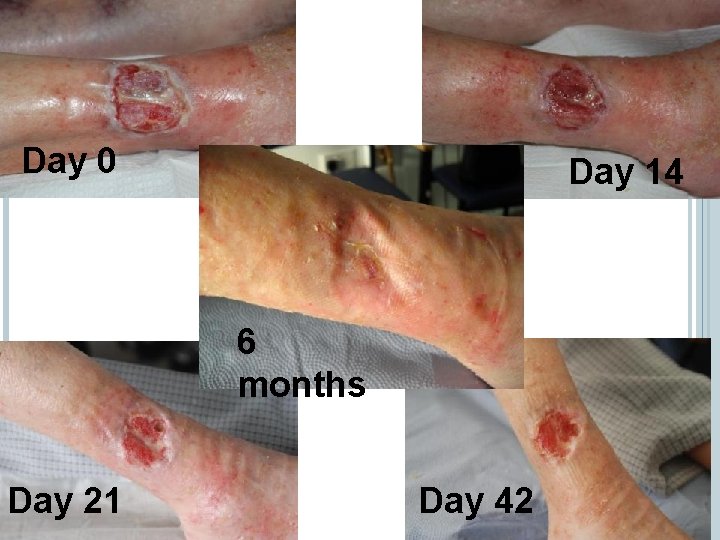
SUCCESSES Day 0 Day 14 6 months Day 21 Day 42

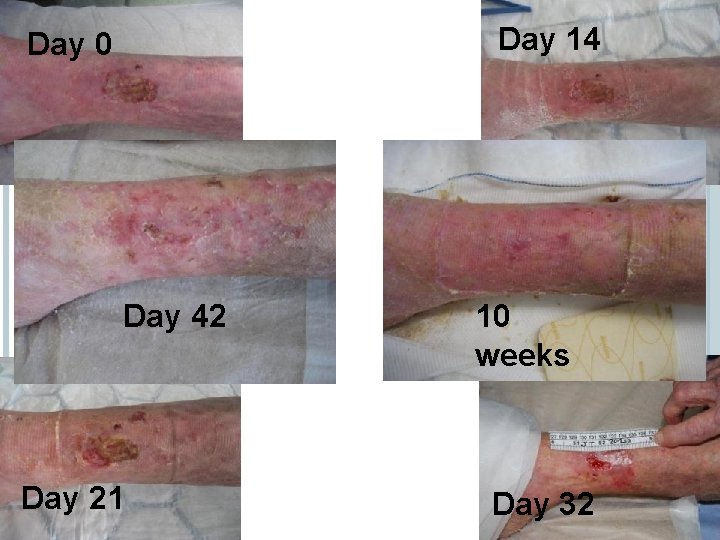
Day 14 Day 0 SUCCESSES Day 42 Day 21 10 weeks Day 32

CONCLUSIONS Skin substitutes have a growing evidence base in the management of chronic wounds and leg ulcers. NHSBT acellular dermis has shown promising early results in this pilot trial. Application is simple and can be performed in a clinic environment. Use of ACD can be combined with compression therapy.

MANY THANKS Any Questions?
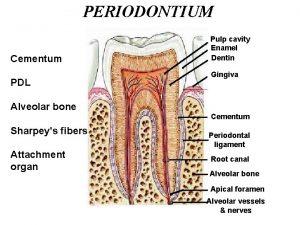
 Acellular cementum
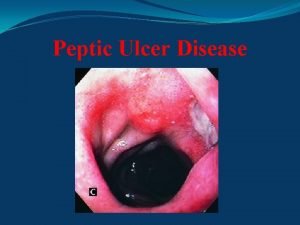
Acellular cementum Stomach ulcer anatomy
Stomach ulcer anatomy Tuberculous ulcer and typhoid ulcer
Tuberculous ulcer and typhoid ulcer Gastric ucler
Gastric ucler Ulcer niche and ulcer notch
Ulcer niche and ulcer notch Parts of an ulcer
Parts of an ulcer Duodenal ulcer anatomy
Duodenal ulcer anatomy Hypotenuse leg congruence theorem
Hypotenuse leg congruence theorem Mamma non lactans
Mamma non lactans Dermis
Dermis Skin dermis
Skin dermis Papillary dermis
Papillary dermis Su içene yılan bile dokunmaz doğrusu tdk
Su içene yılan bile dokunmaz doğrusu tdk Stratum corneum
Stratum corneum Layer of tissue
Layer of tissue Which letter indicates the dermis
Which letter indicates the dermis Uneven junction of the dermis with the epidermis
Uneven junction of the dermis with the epidermis Estrato basal
Estrato basal Narrow cavity in the dermis from which hair cells grow
Narrow cavity in the dermis from which hair cells grow Slide
Slide Streptekok
Streptekok Dermis
Dermis Stratum lucidum คือ
Stratum lucidum คือ