The Story of MTA 02 Stephen Senn SJS















- Slides: 28

The Story of MT/A 02 Stephen Senn SJS Dundonald Road The Story of MTA 02 1

Background • Formoterol is a long-acting extremely potent beta-agonist used in the treatment of asthma. Originally patented by Yamanouchi, who, however only developed an oral form, it was licensed in the mid 1980 s to CIBA-Geigy. • At the time of my arrival at, C-G Basle in 1987 it had just been scheduled for international development in solution form delivered by metered-dose inhaler. • In the course of the next few years various other formulations: suspension, single-dose dry-powder inhaler and multi-dose inhaler were developed. • MTA/02 was a trial designed as part of a programme to show equivalence of a new multi-dose dry powder form (MT&A) to an existing single-dose form (ISF). SJS Dundonald Road The Story of MTA 02 2

SJS Dundonald Road 3

Context • Many trials had been run with formoterol solution – This formulation could, however, only be kept stable using a cold chain for delivery and was not widely marketed. • The suspension formulation had been abandoned because creaming tendency made it too potent. • The dry powder ISF formulation was a technical success but required priming anew every time it was used. • A multi-dose formulation was desirable from the patient and marketing point of view. • There was no desire from the company’s point of view to start all over again. Hence an equivalence route was sought. SJS Dundonald Road The Story of MTA 02 4

Bioequivalence of two bronchodilators given by inhalation • Bronchodilators are inhaled hence classical bioequivalence impossible. • A pharmacodynamic solution is necessary. • Standard bronchodilator doses are very often on flat part of dose-response curve. • Hence what is really required is a parallel dose assay. SJS Dundonald Road The Story of MTA 02 5

Problems • Three doses of test and reference were required. • Placebo should also be included. • Patients could not be treated more than 5 times. • Very high precision was required. • This made a parallel group trial unattractive. SJS Dundonald Road The Story of MTA 02 6

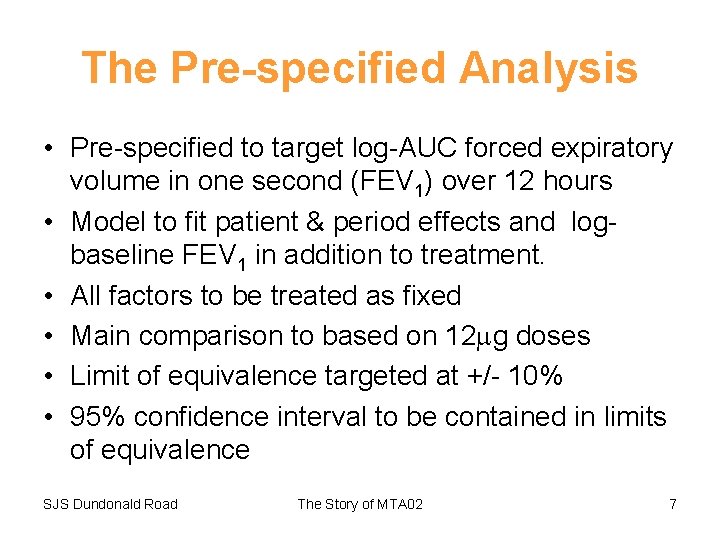
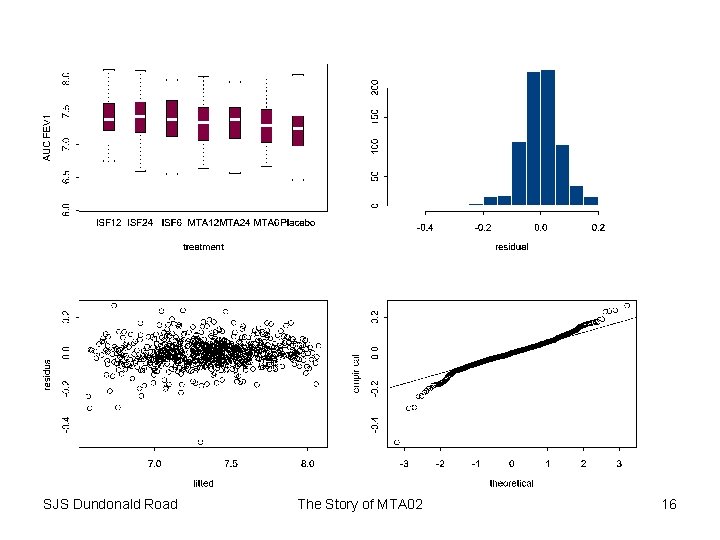
The Pre-specified Analysis • Pre-specified to target log-AUC forced expiratory volume in one second (FEV 1) over 12 hours • Model to fit patient & period effects and logbaseline FEV 1 in addition to treatment. • All factors to be treated as fixed • Main comparison to based on 12 g doses • Limit of equivalence targeted at +/- 10% • 95% confidence interval to be contained in limits of equivalence SJS Dundonald Road The Story of MTA 02 7

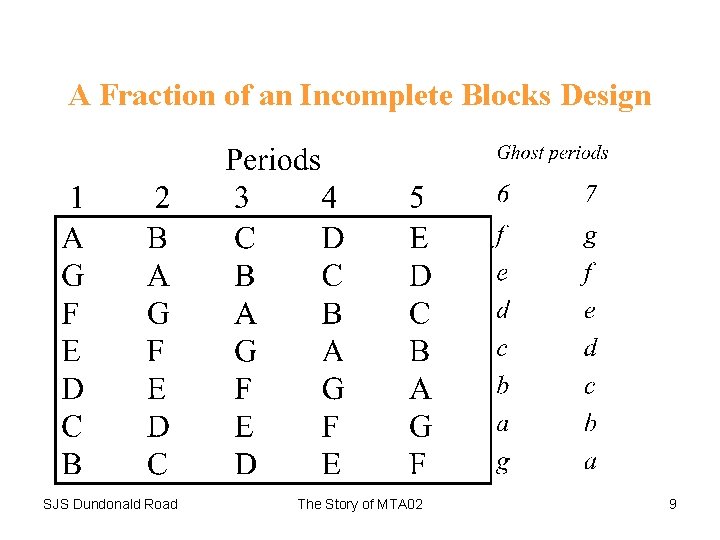
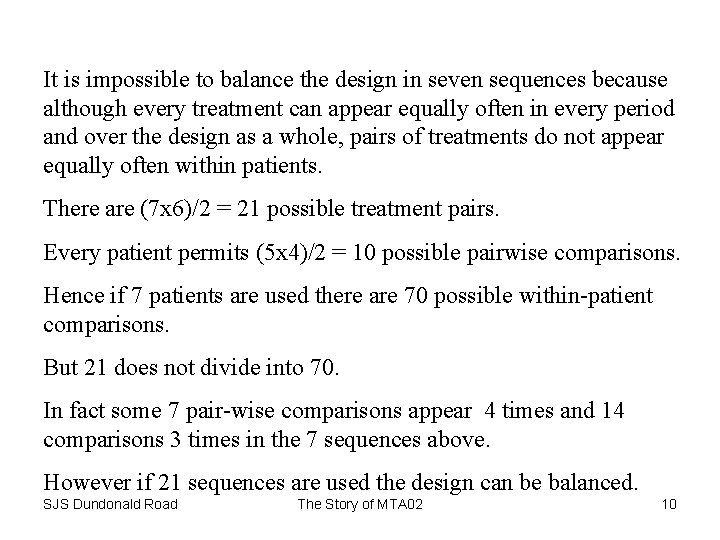
Solution • Incomplete blocks design in seven treatments (three doses test, three doses reference and placebo) and five periods. • This required twenty-one sequences in order to balance treatments. • Sequences to be replicated 6 times = 126 patients. • Trial run in over a dozen centres. SJS Dundonald Road The Story of MTA 02 8

A Fraction of an Incomplete Blocks Design SJS Dundonald Road The Story of MTA 02 9

It is impossible to balance the design in seven sequences because although every treatment can appear equally often in every period and over the design as a whole, pairs of treatments do not appear equally often within patients. There are (7 x 6)/2 = 21 possible treatment pairs. Every patient permits (5 x 4)/2 = 10 possible pairwise comparisons. Hence if 7 patients are used there are 70 possible within-patient comparisons. But 21 does not divide into 70. In fact some 7 pair-wise comparisons appear 4 times and 14 comparisons 3 times in the 7 sequences above. However if 21 sequences are used the design can be balanced. SJS Dundonald Road The Story of MTA 02 10

Some People Involved • Denise Till – project statistician formoterol • Francesco Patalano – medical advisor • Stephen Senn – group leader IBA ex project statistician • Jürgen Lillienthal head of Datamap, the CRO analysing the trial SJS Dundonald Road The Story of MTA 02 11

SJS Dundonald Road The Story of MTA 02 12

SJS Dundonald Road The Story of MTA 02 13

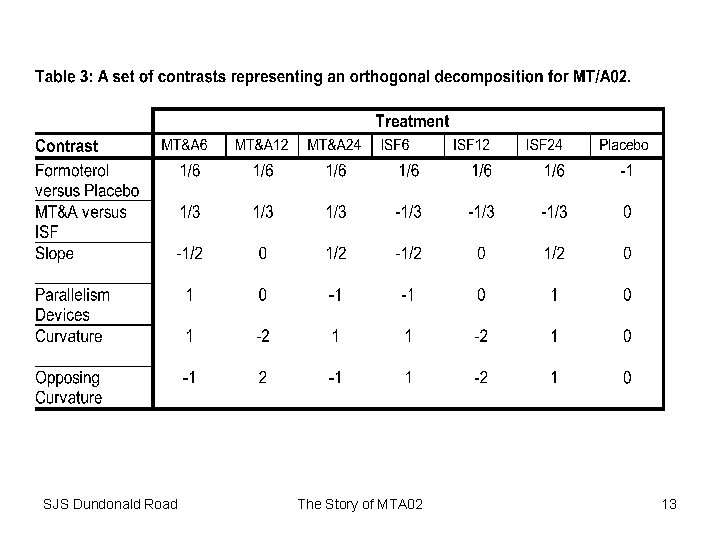
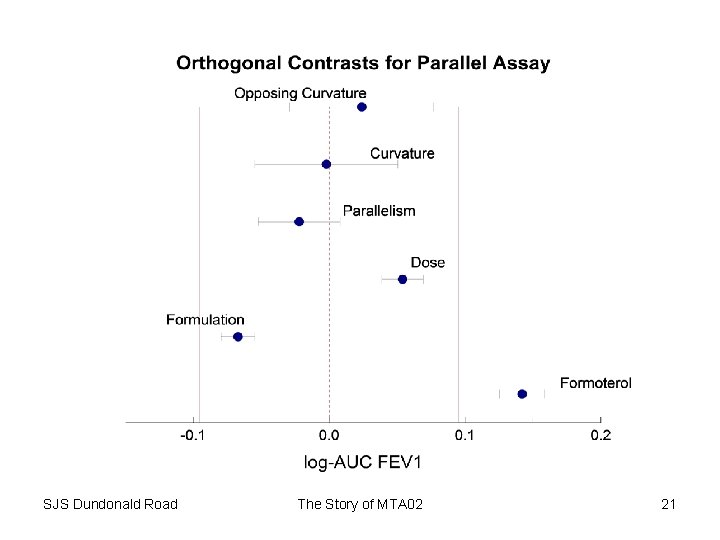
Fixed Effects Analysis * run a fixed effects analysis of the data; Title 2 'Fixed patient effects'; proc glm data=incomp; class TREAT PERIOD PATIENT; model AUC=PATIENT BASE PERIOD TREAT; estimate "MTA 6" TREAT 0 0 0 1 -1 ; estimate "MTA 12" TREAT 0 0 0 1 0 0 -1 ; estimate "MTA 24" TREAT 0 0 1 0 -1 ; estimate "ISF 6" TREAT 0 0 1 0 0 0 -1 ; estimate "ISF 12" TREAT 1 0 0 0 -1 ; estimate "ISF 24" TREAT 0 1 0 0 -1 ; estimate "treatment" TREAT 1 1 1 -6 / divisor=6; estimate "formulation" TREAT -1 -1 -1 1 0/ divisor=3; estimate "dose" TREAT 0 1 -1 0/divisor=2; estimate "parallelism" TREAT 0 1 -1 0 -1 1 0; estimate "curvature" TREAT -2 1 1 0; estimate "opposing curvature" TREAT -2 1 1 2 -1 -1 0; run; The Story of MTA 02 14

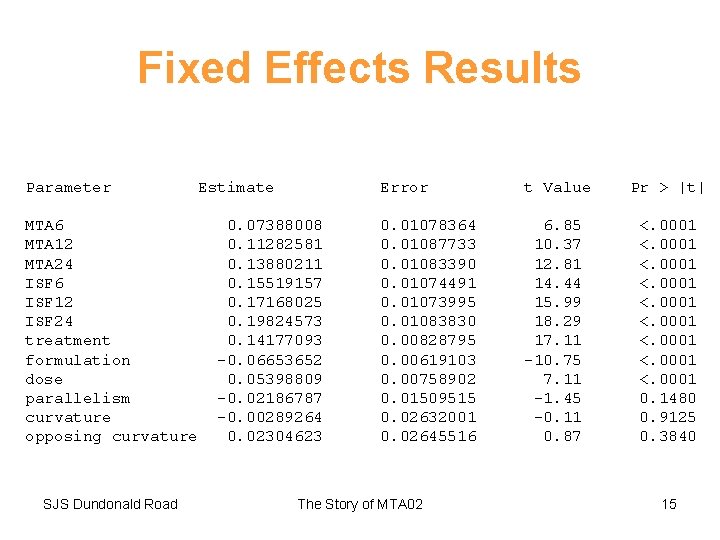
Fixed Effects Results Parameter MTA 6 MTA 12 MTA 24 ISF 6 ISF 12 ISF 24 treatment formulation dose parallelism curvature opposing curvature SJS Dundonald Road Estimate 0. 07388008 0. 11282581 0. 13880211 0. 15519157 0. 17168025 0. 19824573 0. 14177093 -0. 06653652 0. 05398809 -0. 02186787 -0. 00289264 0. 02304623 Error t Value Pr > |t| 0. 01078364 0. 01087733 0. 01083390 0. 01074491 0. 01073995 0. 01083830 0. 00828795 0. 00619103 0. 00758902 0. 01509515 0. 02632001 0. 02645516 6. 85 10. 37 12. 81 14. 44 15. 99 18. 29 17. 11 -10. 75 7. 11 -1. 45 -0. 11 0. 87 <. 0001 <. 0001 0. 1480 0. 9125 0. 3840 The Story of MTA 02 15

SJS Dundonald Road The Story of MTA 02 16

Random Effects Analysis *run a random effects analysis of the data; Title 2 'Random patient effects'; proc mixed data=incomp; class TREAT PERIOD PATIENT; model AUC=BASE PERIOD TREAT; random patient; estimate "MTA 6" TREAT 0 0 0 1 -1 ; estimate "MTA 12" TREAT 0 0 0 1 0 0 -1 ; estimate "MTA 24" TREAT 0 0 1 0 -1 ; estimate "ISF 6" TREAT 0 0 1 0 0 0 -1 ; estimate "ISF 12" TREAT 1 0 0 0 -1 ; estimate "ISF 24" TREAT 0 1 0 0 -1 ; estimate "treatment" TREAT 1 1 1 -6 / divisor=6; estimate "formulation" TREAT -1 -1 -1 1 0/ divisor=3; estimate "dose" TREAT 0 1 -1 0/divisor=2; estimate "parallelism" TREAT 0 1 -1 0 -1 1 0; estimate "curvature" TREAT -2 1 1 0; estimate "opposing curvature" TREAT -2 1 1 2 -1 -1 0; run; SJS Dundonald Road The Story of MTA 02 17

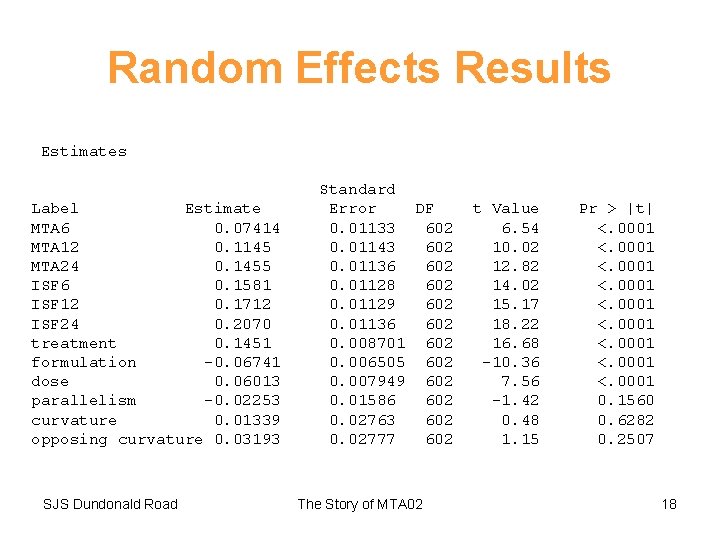
Random Effects Results Estimates Label Estimate MTA 6 0. 07414 MTA 12 0. 1145 MTA 24 0. 1455 ISF 6 0. 1581 ISF 12 0. 1712 ISF 24 0. 2070 treatment 0. 1451 formulation -0. 06741 dose 0. 06013 parallelism -0. 02253 curvature 0. 01339 opposing curvature 0. 03193 SJS Dundonald Road Standard Error DF 0. 01133 602 0. 01143 602 0. 01136 602 0. 01128 602 0. 01129 602 0. 01136 602 0. 008701 602 0. 006505 602 0. 007949 602 0. 01586 602 0. 02763 602 0. 02777 602 The Story of MTA 02 t Value 6. 54 10. 02 12. 82 14. 02 15. 17 18. 22 16. 68 -10. 36 7. 56 -1. 42 0. 48 1. 15 Pr > |t| <. 0001 <. 0001 0. 1560 0. 6282 0. 2507 18

Placebo and the 3 doses of the new formulation SJS Dundonald Road The Story of MTA 02 19

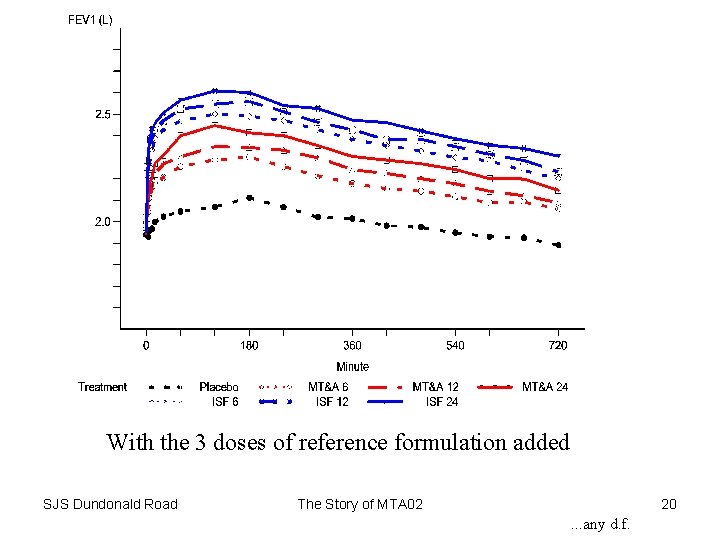
With the 3 doses of reference formulation added SJS Dundonald Road The Story of MTA 02 20 . . . any d. f.

SJS Dundonald Road The Story of MTA 02 21

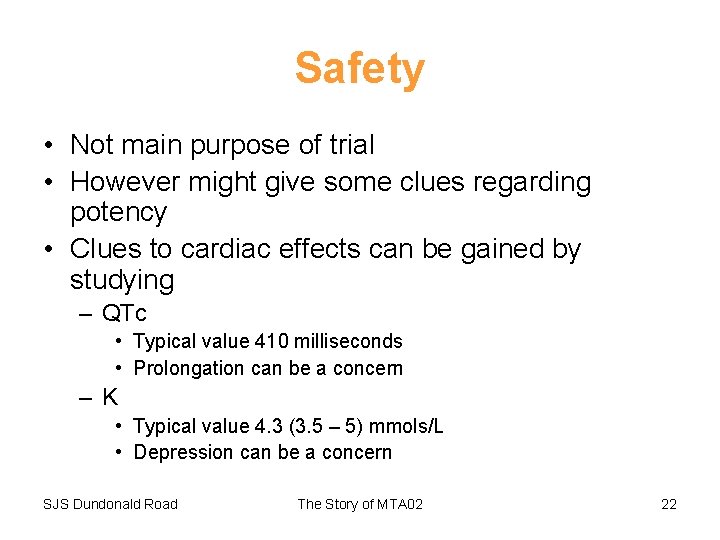
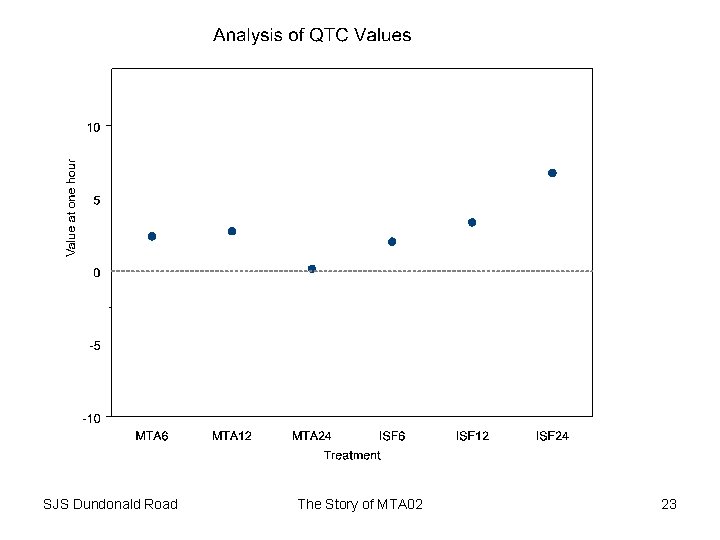
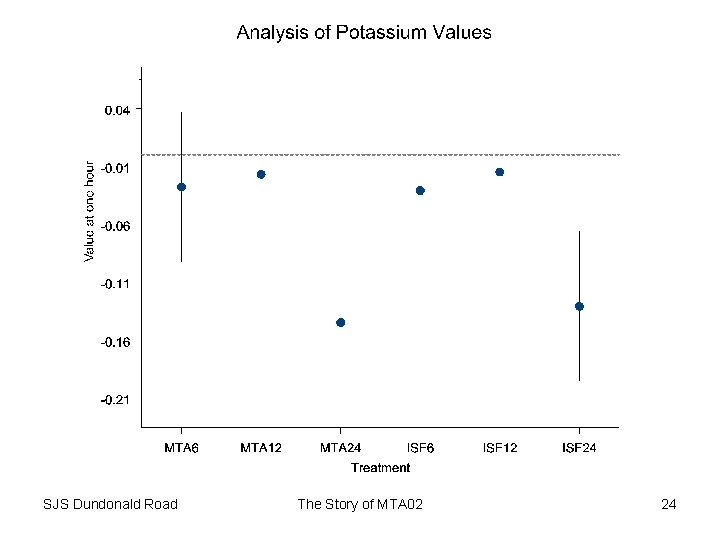
Safety • Not main purpose of trial • However might give some clues regarding potency • Clues to cardiac effects can be gained by studying – QTc • Typical value 410 milliseconds • Prolongation can be a concern –K • Typical value 4. 3 (3. 5 – 5) mmols/L • Depression can be a concern SJS Dundonald Road The Story of MTA 02 22

SJS Dundonald Road The Story of MTA 02 23

SJS Dundonald Road The Story of MTA 02 24

Safety Conclusion • Little evidence of dose-effect for QTc – At least as regards average • Evidence of effect on Potassium at highest doses – Effect small – However, it is interesting that the effect reflects dose delivered rather than potency • Implications for therapeutic ratio SJS Dundonald Road The Story of MTA 02 25

Denouement • The formulation was abandoned – Despite the initial criterion of equivalence being satisfied – MTA Had ¼ the potency of ISF – It was recognised that the original criterion was too lax • The company (now Novartis) continues to market formoterol (Foradil®) ISF and develop new formulations but since the drug is long off patent faces competition from Astra-Zeneca (Oxis®) and generic manufacturers SJS Dundonald Road The Story of MTA 02 26

What would I do differently? • Look at equivalence in terms of dose-scale rather than response scale – Fieller’s theorem • Plus or minus 20% traditional on dose scale but unatainable using FEV 1 and bronchodilators • Decision-analytic approach? – Will the regulator agree? • NO! SJS Dundonald Road The Story of MTA 02 27

Relative potency Estimate 0. 18 95% CI 0. 08 -0. 29 SJS Dundonald Road The Story of MTA 02 28
 Angioedema anaphylaxis
Angioedema anaphylaxis Zach osman
Zach osman Dominique senn
Dominique senn Dominique senn
Dominique senn Dominique senn
Dominique senn Tera mta
Tera mta Mta security fundamentals exam questions
Mta security fundamentals exam questions Johnstone supply moore
Johnstone supply moore Ericsson mta 1956
Ericsson mta 1956 Mta bsc self service portal
Mta bsc self service portal Mta classification
Mta classification Dentinal tubules
Dentinal tubules Precision optical blanks
Precision optical blanks Mta kocsi idk
Mta kocsi idk Assa twin combi key cutting
Assa twin combi key cutting Software sts
Software sts Radioactivity
Radioactivity Bts mta
Bts mta Smr code chart
Smr code chart Mta
Mta Mta psychologie
Mta psychologie Mta business center
Mta business center Mta brand
Mta brand Farkas dock
Farkas dock Mta sztaki
Mta sztaki Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Công của trọng lực
Công của trọng lực Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi













































