THE SELF MEDICATION MANUFACTURERS ASSOCIATION OF SOUTH AFRICA






















- Slides: 26

THE SELF MEDICATION MANUFACTURERS ASSOCIATION OF SOUTH AFRICA (SMASA) MEDICINES AND RELATED SUBSTANCES AMENDMENT BILL, [BILL 6 OF 2014] Allison Vienings – Executive Director Dr Deepa Maharaj – Vice Chair – Technical Health Portfolio Committee Cape Town, 29 October 2014 SMASA - Medicines Bill 6 1

Who we are • SMASA was formed in 1999 and represents the interests of the manufactures of non-prescription medicines (OTC) • We serve to promote responsible self-medication to the public • SMASA welcomes and supports the introduction of SAHPRA and Bill 6 • SMASA is appreciative of the opportunity to present to Parliamentary Health Portfolio Committee SMASA - Medicines Bill 6 2

SMASA members Full Members • Smith & Nephew • Adcock Ingram Healthcare • Takeda • Aspen Pharmacare • Vital Health Foods • Bayer Consumer Care • Winthrop Pharmaceuticals (Sanofi Consumer Healthcare) • Boehringer-Ingelheim SA • FDC SA Associate Members • Glaxo. Smith. Kline SA • Clicks Holdings • i. Nova Pharmaceuticals • Dischem Pharmacies • Johnson & Johnson Consumer • Imperial Health Sciences • Merck Consumer Healthcare • Medi. Rite Pharmacies SA • Norgine • (Shoprite Checkers) • Novartis SA • MRA Regulatory Consultants • Pfizer Consumer Healthcare • X-Procure Software SA • Procter & Gamble • Reckitt Benckiser • Sandoz SA SMASA - Medicines Bill 6 3

Discussion points 1. Matters of Principle – Rationalisation required – Alignment and harmonization required – Main areas of concern 2. Specific Comment – Definitions : complementary products – Scheduled substances: where to remove – Scheduled substances are not products 2. Recommendation and Final Conclusion SMASA - Medicines Bill 6 4

Matters of Principle SMASA - Medicines Bill 6 5

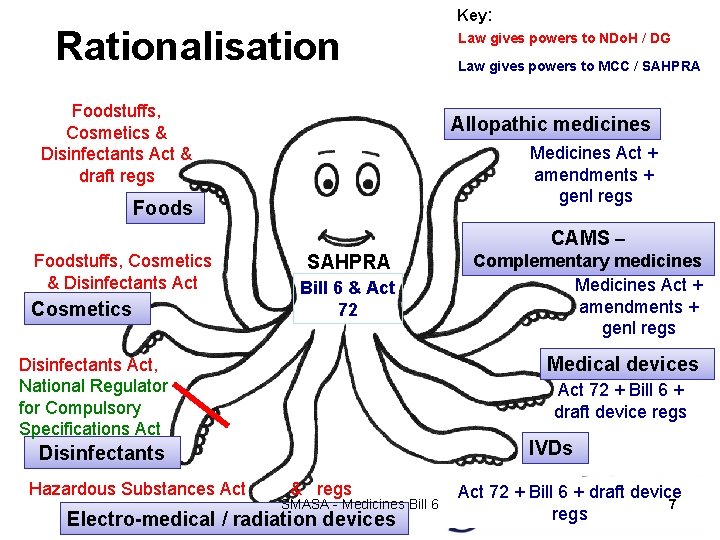
Rationalization required • One size does not fit all – Create within SAHPRA structures that are appropriate for each product type e. g. medicines, cosmetics, medical devices, CAMs – Ensure adequate and fair representation of all products within SAHPRA board, committees and technical structures – Create registration flows and criteria appropriate to the type of product in question SMASA - Medicines Bill 6 6

Rationalisation Foodstuffs, Cosmetics & Disinfectants Act & draft regs Key: Law gives powers to NDo. H / DG Law gives powers to MCC / SAHPRA Allopathic medicines Medicines Act + amendments + genl regs Foods CAMS – Foodstuffs, Cosmetics & Disinfectants Act Cosmetics SAHPRA Bill 6 & Act 72 Complementary medicines Medicines Act + amendments + genl regs Medical devices Disinfectants Act, National Regulator for Compulsory Specifications Act 72 + Bill 6 + draft device regs IVDs Disinfectants Hazardous Substances Act & regs SMASA - Medicines Bill 6 Electro-medical / radiation devices Act 72 + Bill 6 + draft device 7 regs

Rationalisation Required • Learn from past experience: 1. What caused the backlog? 2. How can further backlogs be avoided as scope of control expands? Timelines? 3. SAHPRA as a PFMA entity? 4. Prevention by re-designing how we do things (instead of getting more “who’s”) 5. Incorporation of products without separate regulatory processes and appropriate staffing and structure (CAMs) SMASA - Medicines Bill 6 8

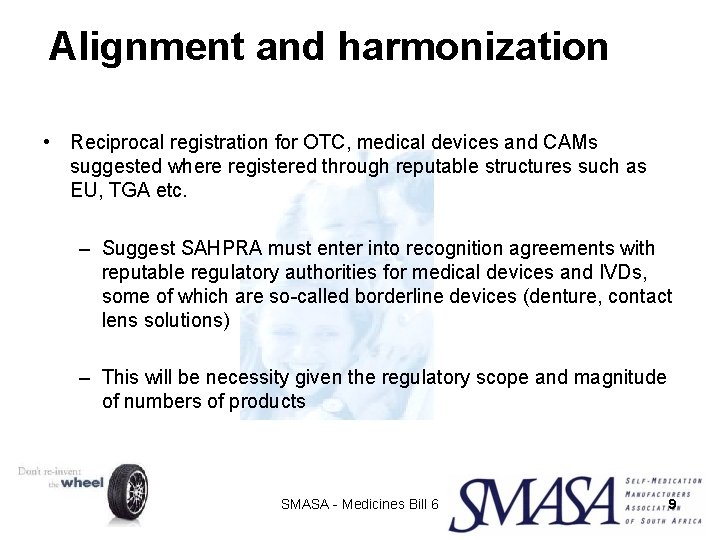
Alignment and harmonization • Reciprocal registration for OTC, medical devices and CAMs suggested where registered through reputable structures such as EU, TGA etc. – Suggest SAHPRA must enter into recognition agreements with reputable regulatory authorities for medical devices and IVDs, some of which are so-called borderline devices (denture, contact lens solutions) – This will be necessity given the regulatory scope and magnitude of numbers of products SMASA - Medicines Bill 6 9

Main Areas of Concern for SMASA • Structure and formation of SAHPRA – skills resources and capacity of SAHPRA to achieve the widest and most efficient form of regulation and control of medicines S. A. - one size (one regulatory model) will not fit all • Timelines associated with registrations and handling of backlog • SAHPRA as a PFMA entity – assurance of financial independence AND financial sustainability • “Regulatory oversight” of Foodstuffs and Cosmetics – Legislative control lies with the Foodstuffs, Cosmetics and Disinfectant Act 54 of 1972. Can mere amendments to the Medicines Act change this? SMASA - Medicines Bill 6 10

Main Areas of Concern for SMASA • To find a solution to the immediate crisis facing the health and wellness sector of industry • Address concerns of the potential damaging effects on the viability of the CAMs industry • Threat of little future availability of health and wellness products for which there is a demand SMASA - Medicines Bill 6 11

Main Areas of Concern for SMASA • Serious consideration must be given to the fact that a “ONE SIZE FITS ALL APPROACH” WILL NOT WORK SMASA - Medicines Bill 6 12

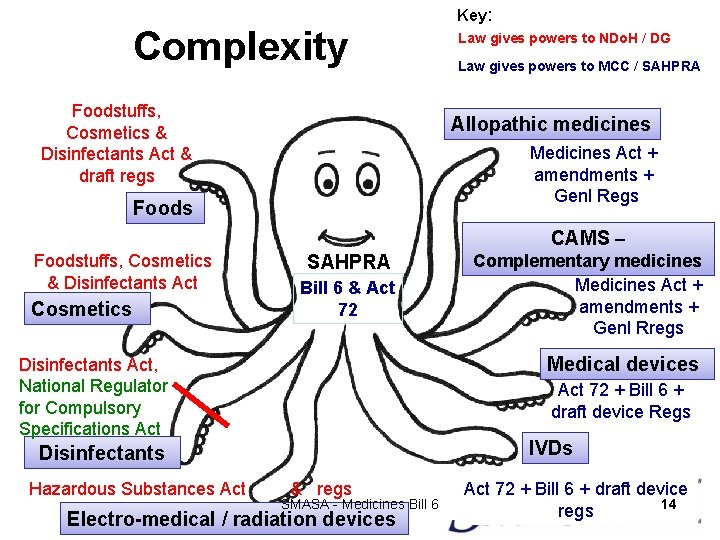
Main Areas of Concern for SMASA • Lack of legal certainty in many areas • The current overlap of regulations has resulted in erroneous categorization and registration of foods, disinfectants, cosmetics and medical devices as medicines • Clear Guidelines needed SMASA - Medicines Bill 6 13

Complexity Foodstuffs, Cosmetics & Disinfectants Act & draft regs Key: Law gives powers to NDo. H / DG Law gives powers to MCC / SAHPRA Allopathic medicines Medicines Act + amendments + Genl Regs Foods CAMS – Foodstuffs, Cosmetics & Disinfectants Act Cosmetics SAHPRA Bill 6 & Act 72 Complementary medicines Medicines Act + amendments + Genl Rregs Medical devices Disinfectants Act, National Regulator for Compulsory Specifications Act 72 + Bill 6 + draft device Regs IVDs Disinfectants Hazardous Substances Act & regs SMASA - Medicines Bill 6 Electro-medical / radiation devices Act 72 + Bill 6 + draft device 14 regs

Specific Comments SMASA - Medicines Bill 6 15

Definition • Definition of a complementary medicine Section 1 (d) “Complementary Medicine” • Inflexible, ill-conceived and misplaced • Should be moved from Principal Act to Regulations • Existing definition of a medicine should stay unchanged • At most the following words should be added: - and includes any veterinary medicine; - biological medicine and - complementary medicine • Definitions for these in Regulation SMASA - Medicines Bill 6 • Definition needed for ‘food supplement’ 16

Definitions • We propose the below definition to be adopted in the regulation “Complementary medicine” means any substance or mixture of substances that(a) originates from, but not limited to plants, fungi, algae, seaweed, lichens, minerals, animals and includes one or more substances included in the substance list as determined by the Council; or other substance as determined by Council (b) is used or purporting to be suitable for use or manufactured or sold for use— (i) in maintaining, complementing, or assisting the innate healing power or Note: Disciplines physical or mental state, or (ii) to diagnose, treat, mitigate, modify, alleviate or prevent disease or illness are problematic… or the symptoms or signs thereof or abnormal physical or mental state of a e. g. rooibos human being or animal, and products relate to (iii) restoring or correcting organic functions in humans, or which discipline? (c) is used(i) as a health supplement (ii) in accordance with those disciplines as determined by Council, or (d) is declared by the Minister, on recommendation by the Council, by notice in the Gazette to be a complementary medicine • In addition a substance list to be published SMASA - Medicines Bill 6 17

“Complementary Medicine” Homeopathic products Glucosamine Plant extracts Pr o an d pr eb io t ic s Vitamin and minerals SMASA - Medicines Bill 6 18

Definitions Examples of Borderline Medical Devices Non corrective contact lens and contact lens solutions - includes daily disposable, daily wear and continuous or extended wear are medical devices Therapeutic clothes e. g. stockings – medical device Certain eye drops - specifically intended to be used for disinfecting, cleaning, rinsing or, when appropriate, hydrating contact lenses are medical devices. Teeth whiteners e. g. toothpaste, whitening strips – cosmetics. Personal protective equipment e. g. masks, shoe covers – medical devices SMASA - Medicines Bill 6 19

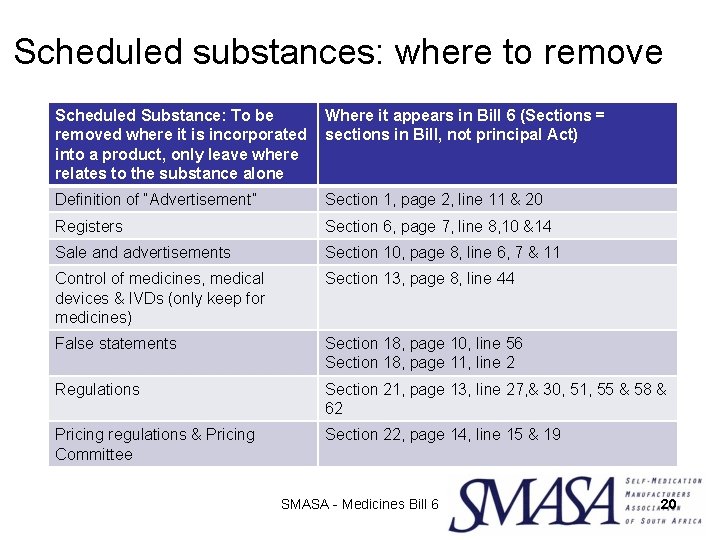
Scheduled substances: where to remove Scheduled Substance: To be removed where it is incorporated into a product, only leave where relates to the substance alone Where it appears in Bill 6 (Sections = sections in Bill, not principal Act) Definition of “Advertisement” Section 1, page 2, line 11 & 20 Registers Section 6, page 7, line 8, 10 &14 Sale and advertisements Section 10, page 8, line 6, 7 & 11 Control of medicines, medical devices & IVDs (only keep for medicines) Section 13, page 8, line 44 False statements Section 18, page 10, line 56 Section 18, page 11, line 2 Regulations Section 21, page 13, line 27, & 30, 51, 55 & 58 & 62 Pricing regulations & Pricing Committee Section 22, page 14, line 15 & 19 SMASA - Medicines Bill 6 20

Scheduled substances are not products Cosmetic (Toothpaste) Fluoride (Scheduled substance) Medical Device (fluoride trays) Complementary medicine (Multivit with Fluoride) Medicine (Fluoride tablets) SMASA - Medicines Bill 6 21

Scheduled substances are not products Cosmetic (Cream) Vitamin A (Scheduled substance) Complementary medicine (Supplement) Food supplement? Medicine (Vitamin A injection) SMASA - Medicines Bill 6 22

Transitional Measures • Remove products erroneously registered as medicines from the register • Pre-assessment of regulatory scope required to enable SAHPRA to plan adequately • A clause is needed for all medical devices, cosmetics and foodstuffs that are not registered but on the market to be grand-fathered to allow market access and business continuity. • Industry should be given 3 years to comply with regulatory requirements SMASA - Medicines Bill 6 23

Recommendations and final conclusions • SMASA welcomes and supports an enhanced regulatory framework and urges the NDo. H to consider existing frameworks in mature authorities – For example, Health Canada’s approach to the regulation of Natural Health Products use of monographs – UK MHRA’s approach to the regulation of medical devices and borderline medical devices such as EU Manual on Borderline and Classification in the Community Regulatory framework for MD (July 2016) • SMASA is happy to partner with NDo. H to ensure a legal framework that is appropriate, and an efficiently functioning SAHPRA SMASA - Medicines Bill 6 24

Contact details Allison Vienings E-mail: smasa. cc@gmail. com Tel: 012 803 6223 www. smasa. cc Thank you SMASA - Medicines Bill 6 25

SMASA - Medicines Bill 6 26
 European diagnostic manufacturers association
European diagnostic manufacturers association Builders hardware manufacturers association
Builders hardware manufacturers association Metal clad cable installation
Metal clad cable installation Plastics machinery manufacturers association of india
Plastics machinery manufacturers association of india Indian pharma association
Indian pharma association Astro foil r value
Astro foil r value Are you an ideal self or a self
Are you an ideal self or a self Agrement south africa
Agrement south africa President team herbalife salary
President team herbalife salary South africa unemployment rate by race
South africa unemployment rate by race Dr hamilton le
Dr hamilton le Unjustified enrichment south africa
Unjustified enrichment south africa Southwest asia
Southwest asia 11 official languages of south africa
11 official languages of south africa Musket
Musket How many capital cities in south africa
How many capital cities in south africa Fundal height by week
Fundal height by week Oxford university press south africa
Oxford university press south africa National core standards checklists
National core standards checklists Naairs south africa
Naairs south africa Ibm client center
Ibm client center Imperialism in africa timeline
Imperialism in africa timeline Zero hunger programme south africa
Zero hunger programme south africa Cultural geography of africa
Cultural geography of africa Neuropsychology in south africa
Neuropsychology in south africa Coastal region south africa
Coastal region south africa Banc plus visits
Banc plus visits