The Respiratory Chain Dr M Azhar Chishti Dept

















- Slides: 27

The Respiratory Chain Dr. M. Azhar Chishti Dept. Medical Biochemistry

Objective 1. To correlate between the oxidation of food molecules by cellular respiration and the mitochondrial extraction of electrons to produce ATP 2. To understand that the mitochondrial electron transport chain provides a mean of using the reducing potentials to produce ATP.

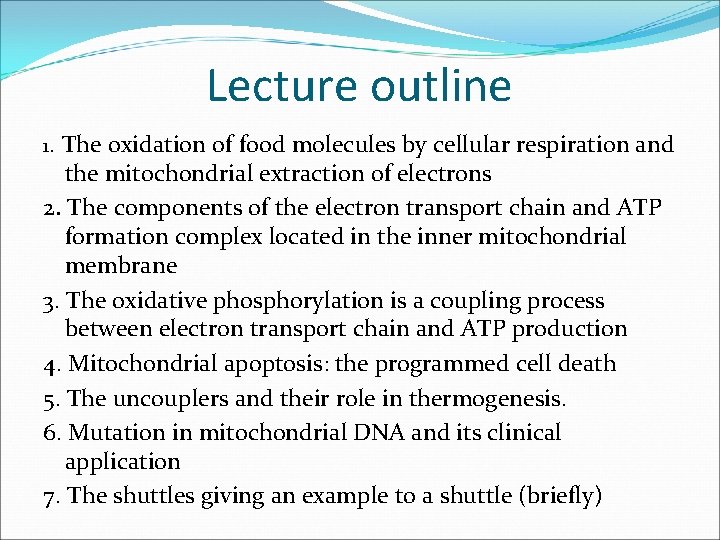
Lecture outline 1. The oxidation of food molecules by cellular respiration and the mitochondrial extraction of electrons 2. The components of the electron transport chain and ATP formation complex located in the inner mitochondrial membrane 3. The oxidative phosphorylation is a coupling process between electron transport chain and ATP production 4. Mitochondrial apoptosis: the programmed cell death 5. The uncouplers and their role in thermogenesis. 6. Mutation in mitochondrial DNA and its clinical application 7. The shuttles giving an example to a shuttle (briefly)

Respiratory Chains Energy-rich molecules, such as glucose, are metabolized by a series of oxidation reactions ultimately yielding ﺑﺎﻟﻨﻬﺎﻳﻪ ﺗﻌﻄﻲ CO 2 and water. The metabolic intermediates of these reactions donate electrons to specific coenzymes— Nicotinamide adenine dinucleotide (NAD+) and flavin adenine dinucleotide (FAD)— These form the energy-rich reduced coenzymes, NADH and FADH 2.

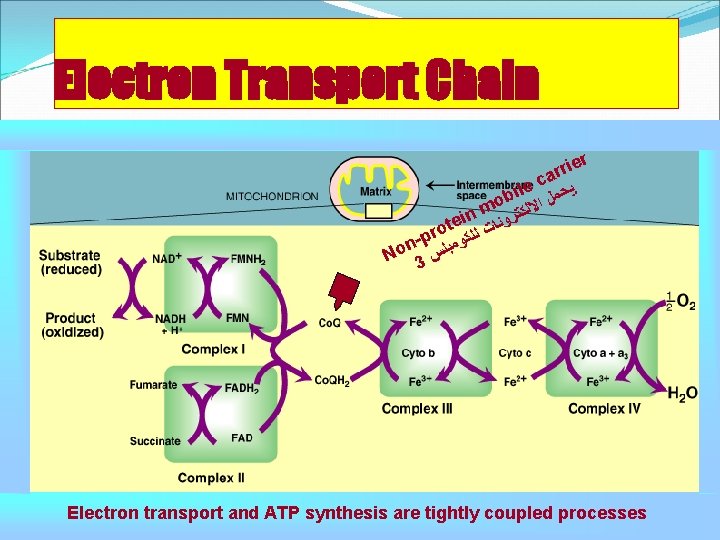
Electron Transport Chain ier r r ca ﻳ e l bi ﺤﻤﻞ ﺍ o m ﻻﻟﻜﺘ n i te ﺮﻭﻧﺎﺕ o r ﻟ n-p ﻠﻜﻮﻣﺒﻠ o N 3 ﺲ Electron transport and ATP synthesis are tightly coupled processes

Electron transport chain These reduced coenzymes can, in turn, each donate a pair of electrons to a specialized set of electron carriers, collectively called the electron transport chain. As electrons are passed down the electron transport chain, they lose much of their free energy. Part of this energy can be captured and stored by the production of ATP from ADP and inorganic phosphate (Pi). This process is called oxidative phosphorylation The remainder of the free energy not trapped as ATP is used to drive ancillary reactions such as Ca 2+ transport into mitochondria, and to generate heat. ﻣﻔﻴﺪﻩ ﻟﺘﺪﻓﺌﻪ ﺍﻟﺠﺴﻢ

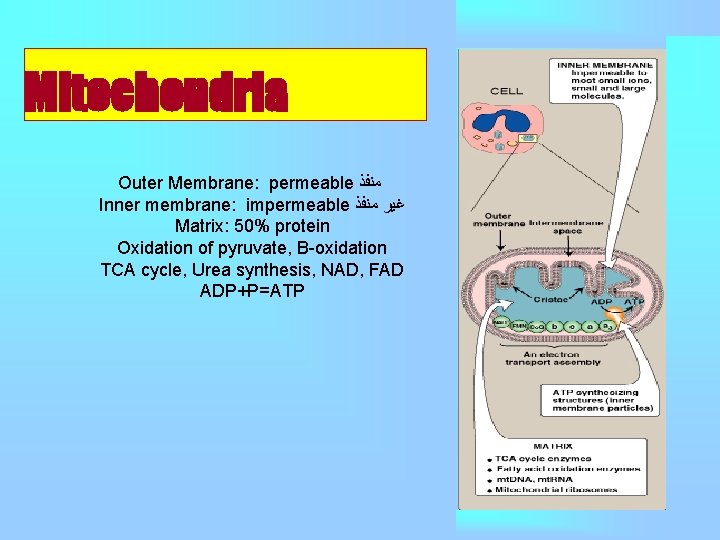
Mitochondria Outer Membrane: permeable ﻣﻨﻔﺬ Inner membrane: impermeable ﻏﻴﺮ ﻣﻨﻔﺬ Matrix: 50% protein Oxidation of pyruvate, Β-oxidation TCA cycle, Urea synthesis, NAD, FAD ADP+P=ATP

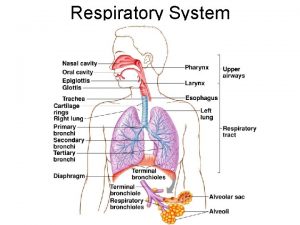
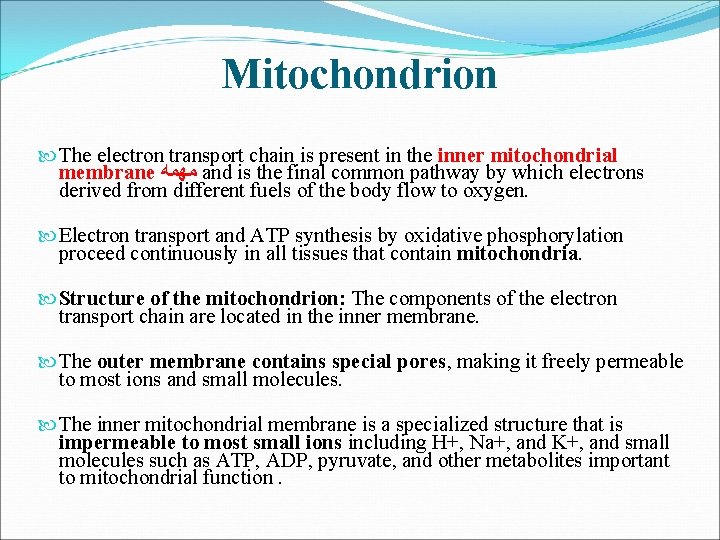
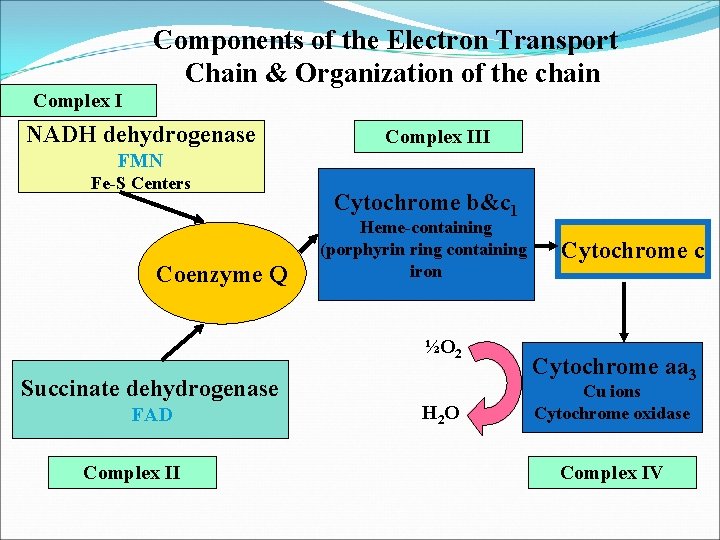
Mitochondrion The electron transport chain is present in the inner mitochondrial membrane ﻣﻬﻤﻪ and is the final common pathway by which electrons derived from different fuels of the body flow to oxygen. Electron transport and ATP synthesis by oxidative phosphorylation proceed continuously in all tissues that contain mitochondria. Structure of the mitochondrion: The components of the electron transport chain are located in the inner membrane. The outer membrane contains special pores, making it freely permeable to most ions and small molecules. The inner mitochondrial membrane is a specialized structure that is impermeable to most small ions including H+, Na+, and K+, and small molecules such as ATP, ADP, pyruvate, and other metabolites important to mitochondrial function.

Specialized carriers or transport systems are required to move ions or molecules across this membrane. ATP synthase complexes: These complexes of proteins contain domains that span ﺟﺴﺮ the inner mitochondrial membrane, and domains that appear as spheres that protrude into the mitochondrial matrix. Matrix of the mitochondrion: This gel-like solution in the interior of mitochondria is 50% percent protein. These molecules include the enzymes responsible for the oxidation of pyruvate, amino acids, fatty acids (by β-oxidation), and those of the tricarboxylic acid (TCA) cycle. The synthesis of glucose, urea, and heme occur partially in the matrix of mitochondria. In addition, the matrix contains NAD+ and FAD (the oxidized forms of the two coenzymes that are required as hydrogen acceptors) and ADP and Pi, which are used to produce ATP. [Note: The matrix also contains mitochondrial RNA and DNA (mt. RNA and mt. DNA) and mitochondrial ribosomes. ]

Electron Transport Chain


Organization of the chain The inner mitochondrial membrane can be disrupted into five separate protein complexes, called complexes I, III, IV, and V. Complexes I to IV each contain part of the electron transport chain. Each complex accepts ﻳﺘﻠﻘﻰ or donates ﻳﺮﺳﻞ electrons to relatively mobile electron carriers, such as coenzyme Q and cytochrome c. Each carrier in the electron transport chain can receive electrons from an electron donor, and can subsequently donate electrons to the next carrier in the chain. The electrons ultimately combine with oxygen and protons to form water. This requirement for oxygen makes the electron transport process the respiratory chain, which accounts for the greatest portion of the body's use of oxygen.

Components of the Electron Transport Chain & Organization of the chain Complex I NADH dehydrogenase Complex III FMN Fe-S Centers Coenzyme Q Cytochrome b&c 1 Heme-containing (porphyrin ring containing iron ½O 2 Succinate dehydrogenase FAD Complex II H 2 O Cytochrome c Cytochrome aa 3 Cu ions Cytochrome oxidase Complex IV

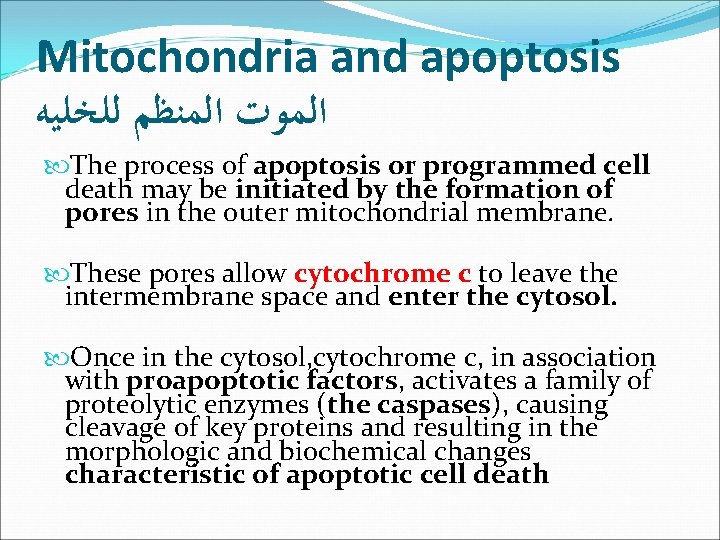
Mitochondria and apoptosis ﺍﻟﻤﻮﺕ ﺍﻟﻤﻨﻈﻢ ﻟﻠﺨﻠﻴﻪ The process of apoptosis or programmed cell death may be initiated by the formation of pores in the outer mitochondrial membrane. These pores allow cytochrome c to leave the intermembrane space and enter the cytosol. Once in the cytosol, cytochrome c, in association with proapoptotic factors, activates a family of proteolytic enzymes (the caspases), causing cleavage of key proteins and resulting in the morphologic and biochemical changes characteristic of apoptotic cell death

Oxidative Phosphorylation Chemiosmotic Hypothesis


Proton pump: Electron transport is coupled to the phosphorylation of ADP by the transport (“pumping”) of protons (H+) across the inner mitochondrial membrane from the matrix to the intermembrane space. The energy generated by this proton gradient is sufficient to drive ATP synthesis. The proton gradient serves as the common intermediate that couples oxidation to phosphorylation. ATP synthase: The enzyme complex ATP synthase (complex V) synthesizes ATP, using the energy of the proton gradient generated by the electron transport chain.


Uncoupling proteins (UCP) ﻣﻬﻤﻪ UCP 1, also called thermogenin , is responsible for the activation of fatty acid oxidation and heat production in the brown adipocytes of mammals.

Inherited defects in oxidative phosphorylation Defects in oxidative phosphorylation are more likely a result of alterations in mt. DNA ﺍﻟﺪﻱ ﺍﻥ ﺍﻱ ﺍﻟﺨﺎﺹ ﺑﺎﻟﻤﻴﺘﻮﻛﻨﺪﺭﻳﺎ , which has a mutation rate about ten times greater than that of nuclear DNA. Mutations in mt. DNA are responsible for several diseases, including some cases of mitochondrial myopathies and Leber hereditary optic neuropathy. The mt. DNA is maternally ﺍﻻﻡ ﻗﺎﺩﻡ ﻣﻦ inherited because mitochondria from the sperm cell do not enter the fertilized egg.


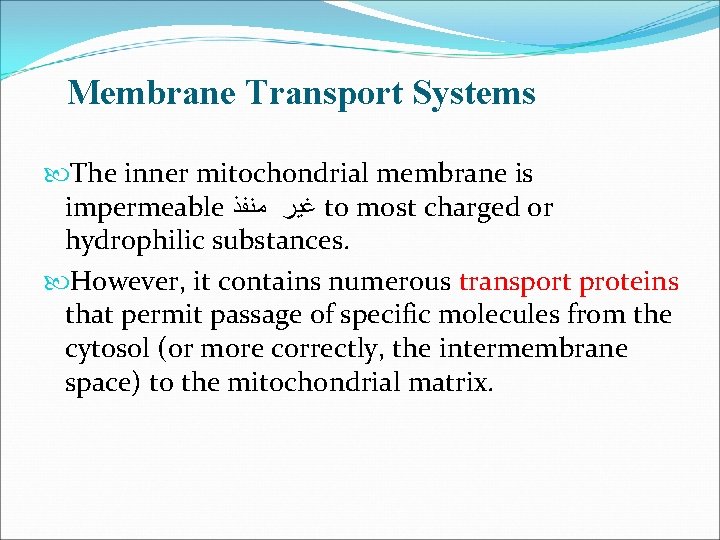
Membrane Transport Systems The inner mitochondrial membrane is impermeable ﻏﻴﺮ ﻣﻨﻔﺬ to most charged or hydrophilic substances. However, it contains numerous transport proteins that permit passage of specific molecules from the cytosol (or more correctly, the intermembrane space) to the mitochondrial matrix.




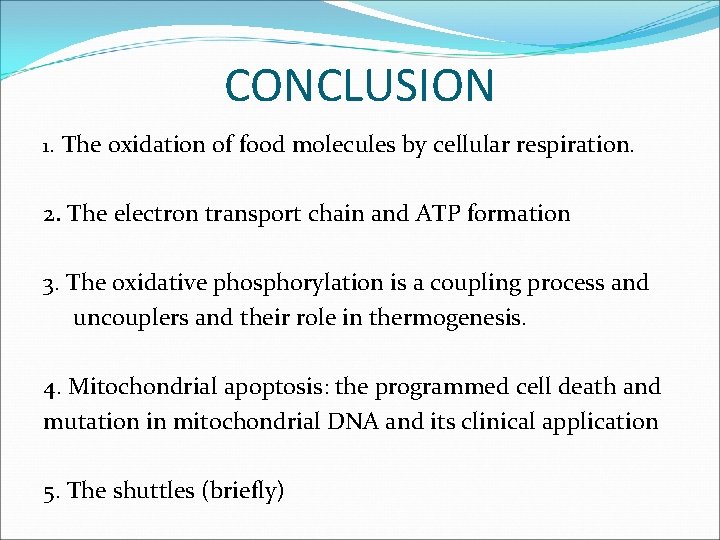
CONCLUSION 1. The oxidation of food molecules by cellular respiration. 2. The electron transport chain and ATP formation 3. The oxidative phosphorylation is a coupling process and uncouplers and their role in thermogenesis. 4. Mitochondrial apoptosis: the programmed cell death and mutation in mitochondrial DNA and its clinical application 5. The shuttles (briefly)

 Compsci 111
Compsci 111 Dr azhar hussain
Dr azhar hussain Salman azhar
Salman azhar Pflng tiga
Pflng tiga Salman azhar
Salman azhar Saiful azhar
Saiful azhar Prof azhar kasim
Prof azhar kasim Discovery learning theory
Discovery learning theory Robert presthus public administration
Robert presthus public administration Eltonian pyramid
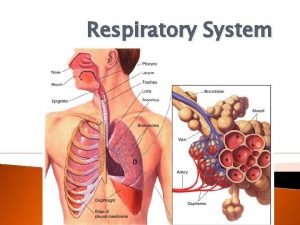
Eltonian pyramid Respiratory zone and conducting zone
Respiratory zone and conducting zone Components of etc
Components of etc Respiratory chain defects
Respiratory chain defects Gome dept
Gome dept Oviposition
Oviposition Dept. name of organization (of affiliation)
Dept. name of organization (of affiliation) Affiliate disclodures
Affiliate disclodures Dept a
Dept a Gome dept
Gome dept Dept. name of organization
Dept. name of organization Dept c13 nmr
Dept c13 nmr Hoe dept
Hoe dept Dept of education
Dept of education Ohio dept of dd
Ohio dept of dd Geaux biz login
Geaux biz login Ee dept iitb
Ee dept iitb Dept nmr spectroscopy
Dept nmr spectroscopy Central islip fire dept
Central islip fire dept