The Pretransplant Evaluation Kidney transplant candidate with PPD



















- Slides: 30

The Pre-transplant Evaluation: Kidney transplant candidate with +PPD A Pediatric Transplant Infectious Diseases Learning Module

Using the Modules • These modules work best if accessed using Google Chrome as your browser • Suggest avoiding Internet Explorer • Some slides contain a code designation tied to the American Board of Pediatrics Content Outline for Pediatric Infectious Diseases, e. g. 3. E. 6 • The basic modules are designed to take about 45 minutes to complete • You might take longer, especially if you choose to investigate all of the informational links provided

Using the Modules, cont’d • The modules are case-based, with decision points (branches) containing questions • Many questions don’t have right or wrong answers • Click on a response (e. g. a diagnostic test), and you will find out more about it • Multiple “action buttons” help you navigate • Take your time and enjoy!

Navigating the Modules • DO NOT use your keyboard arrows or mouse click to advance slides • Only use the navigation buttons on each slide – these will keep you from getting lost • If you do get lost, you can hit the “home” button any time to go back • Unlabeled button types you might encounter: • • • Previous slide Next slide First slide More information Return to decision choices • Other buttons are self-explanatory

Case • 14 y. o. African American male with Eagle Barrett syndrome and end stage renal disease (ESRD) due to obstructive uropathy secondary to posterior uretheral valves (PUV). • He was started on nightly home peritoneal dialysis for approximately 6 months. • He performs clean intermittent catheterization at home through a Mitroffanoff. • Urology has cleared the patient to proceed with transplantation.

History • Past medical and surgical histories: • Eagle Barrett Syndrome • PUV S/P ablation • Neurogenic bladder • S/P Mitroffanoff creation • History of frequent urinary tract infections; resolved since initiation of CIC • S/P Tenckhoff placement • Family Hx: • Hypertension in maternal grandmother • Type 2 DM in paternal aunt • Social History: Lives at home with mother, father, sibling of 8 years. Has a dog and fish. He is currently in 9 th Grade. • ROS: His mother relates that other than his kidney disease he has been well. She denies fevers, cough, ENT concerns, chest pain, vomiting, diarrhea, weight loss, night sweats.

Physical Exam: BP 108/79 | Pulse 102 | Temp 96. 8 (Tympanic)|Ht 153 cm (5%ile) cm | Wt 42 kg (10%ile) General: Well-appearing, interactive and talkative SKIN: normal, no rashes HEENT: NC/AT, normal, moist mucus membranes, braces in place NECK: supple, no LAD LUNGS: Clear to auscultation bilaterally, no crackles or wheezes, good aeartion HEART: Regular rate and rhythm; normal S 1 and S 2; no murmurs, gallops, or rubs. Strong peripheral pulses. ABD: soft, non-tender, skin around Mitroffanoff clean and dry, decreased tone and musculature, Tenckhoff in place – clean and dry MUSCULOSKELETAL: normal EDEMA: absent

2. M. 1. b Tuberculosis Risk factors assessment: • Recent visit for 3 weeks from Grandmother from Nigeria. • Grandmother had cough and stayed in the patient house during visit. • Returned to Nigeria 4 weeks prior to evaluation. 1. Pediatric Tuberculosis Collaborative Group. Targeted Tuberculin Skin Testing and Treatment of Latent Tuberculosis Infection in Children and Adolescents. Pediatrics 2004; 114: 1175 -1201. 2. AAP Red Book 2015. Table 3. 80 Validated Questions for Determining Risk of LTBI in Children in the United States.

Initial Tests? Review of Vaccine Record Infectious Disease Serology PPD Placement

Vaccine Record • Vaccines Up To Date for age based on vaccine record review • Has received annual Influenza vaccine

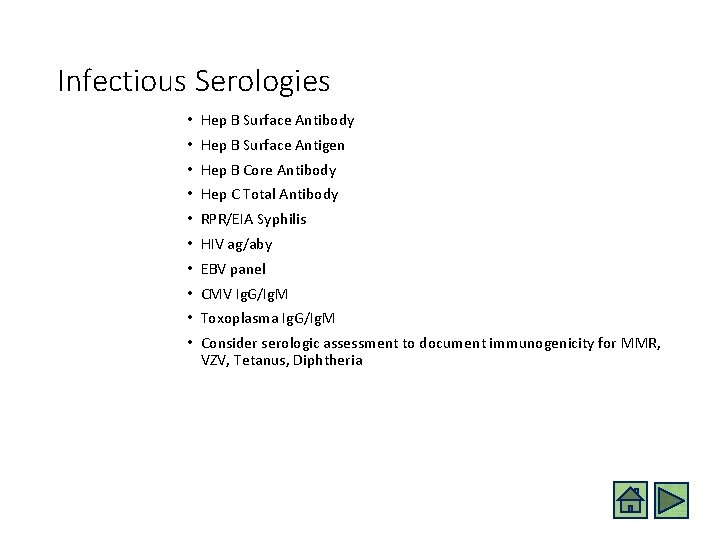
Infectious Serologies • Hep B Surface Antibody • Hep B Surface Antigen • Hep B Core Antibody • Hep C Total Antibody • RPR/EIA Syphilis • HIV ag/aby • EBV panel • CMV Ig. G/Ig. M • Toxoplasma Ig. G/Ig. M • Consider serologic assessment to document immunogenicity for MMR, VZV, Tetanus, Diphtheria

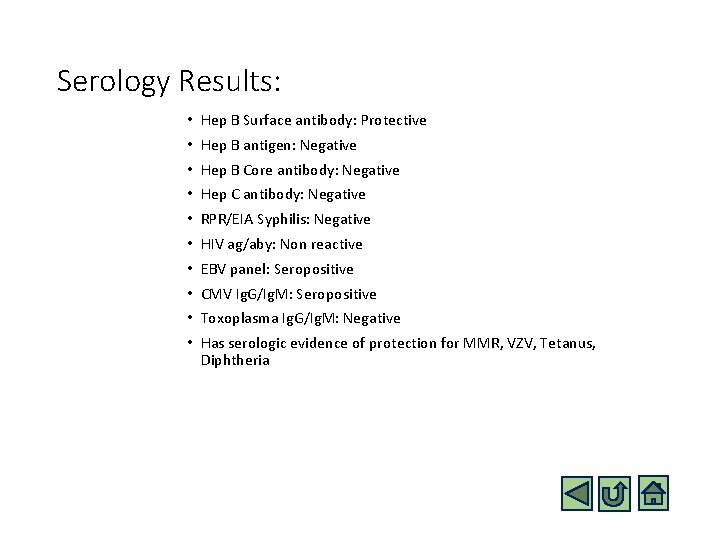
Serology Results: • Hep B Surface antibody: Protective • Hep B antigen: Negative • Hep B Core antibody: Negative • Hep C antibody: Negative • RPR/EIA Syphilis: Negative • HIV ag/aby: Non reactive • EBV panel: Seropositive • CMV Ig. G/Ig. M: Seropositive • Toxoplasma Ig. G/Ig. M: Negative • Has serologic evidence of protection for MMR, VZV, Tetanus, Diphtheria

2. M. 1. f 2. M. 1. s PPD Reading: • PPD placed • Patient returned in 48 hours for reading of PPD • PPD is read as 11 mm at 48 hours • PPD is interpreted as “positive” Subramanian AK, Morris MI. Mycobacterium tuberculosis infections in solid organ transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2013; 13 Suppl 4: 68 -76.

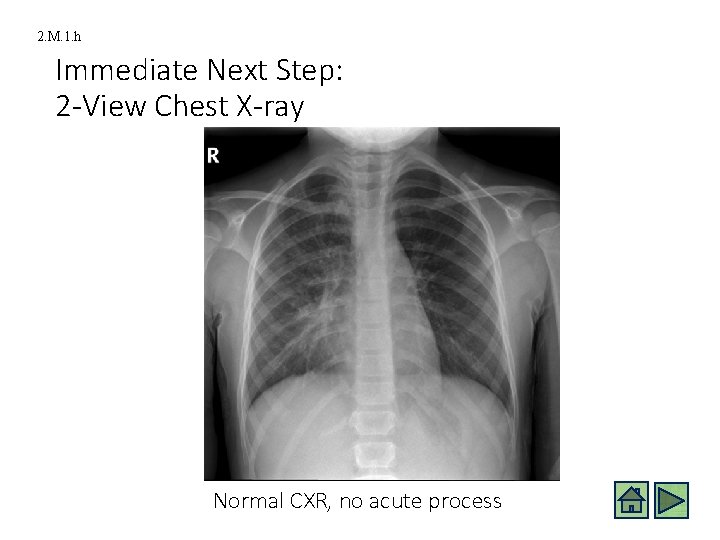
2. M. 1. h Immediate Next Step: 2 -View Chest X-ray Normal CXR, no acute process

2. M. 1. f Peritoneal- and Hemo-dialysis patients are a unique population • Patients with End-Stage Kidney Disease (ESKD) are 6 -25 X more likely to develop TB than the general population (impaired cellular immunity) • Mortality rates from active TB in dialysis populations are high (17 -75%) • Active TB in hemodialysis (HD) patients often has an atypical presentation resulting in delay in diagnosis and therapy • Screening for Latent Tuberculosis Infection (LTBI) is recommended for all ESKD patients Segall L, Covic A. Diagnosis of tuberculosis in dialysis patients: current strategy. Clinical journal of the American Society of Nephrology : CJASN 2010; 5: 1114 -22.

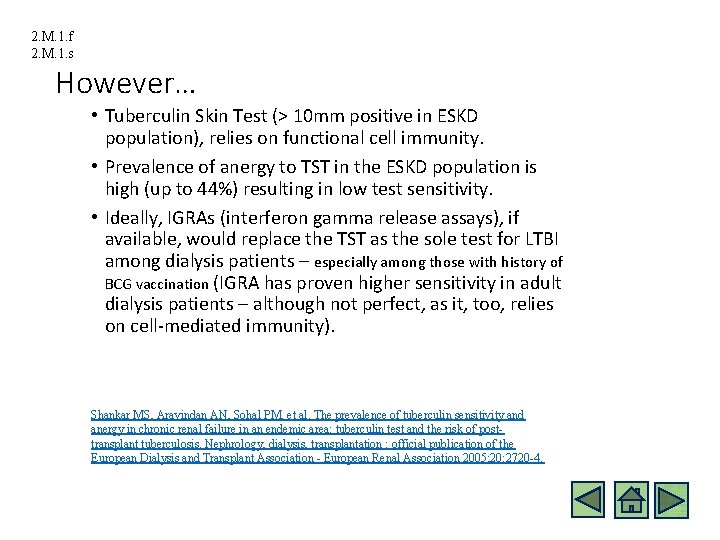
2. M. 1. f 2. M. 1. s However… • Tuberculin Skin Test (> 10 mm positive in ESKD population), relies on functional cell immunity. • Prevalence of anergy to TST in the ESKD population is high (up to 44%) resulting in low test sensitivity. • Ideally, IGRAs (interferon gamma release assays), if available, would replace the TST as the sole test for LTBI among dialysis patients – especially among those with history of BCG vaccination (IGRA has proven higher sensitivity in adult dialysis patients – although not perfect, as it, too, relies on cell-mediated immunity). Shankar MS, Aravindan AN, Sohal PM, et al. The prevalence of tuberculin sensitivity and anergy in chronic renal failure in an endemic area: tuberculin test and the risk of posttransplant tuberculosis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 2005; 20: 2720 -4.

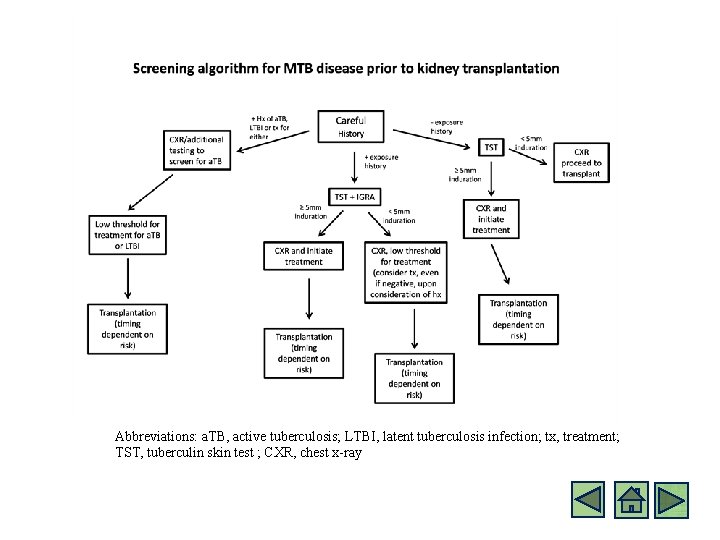
2. M. 1. f Kidney Transplant Preparation: LTBI screening (2013 AST guidelines) • All candidates for Kidney Transplantation should be screened for LTBI (irrespective of whether or not receiving renal replacement therapy) • The most frequent source of TB infection in transplant recipients is reactivation from quiescent foci • Careful history must be taken (including, prior TST results, exposures, travel, prior treatment for TB/LTBI) • Conventional TST is considered positive if >5 mm induration at 48 -72 hours after placement in a potential transplant recipient • If negative initially, repeat in 2 weeks • Consider follow up testing with IGRA • Chest x-ray should be obtained in all transplant candidates • NOTE: “None of the available screening tests are infallible therefore treatment decisions must be individualized based on the clinical likelihood of infection and careful review of the data” 1 Subramanian AK, Morris MI. Mycobacterium tuberculosis infections in solid organ transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2013; 13 Suppl 4: 68 -76.

Abbreviations: a. TB, active tuberculosis; LTBI, latent tuberculosis infection; tx, treatment; TST, tuberculin skin test ; CXR, chest x-ray

Screening for Living/Deceased Donors: • Living donors should undergo evaluation similar to that described for transplant recipient candidates • TST should be interpreted as positive or negative as per CDC guidelines for the general population • If positive TST CXR to r/o active disease, then treat. • “Organs from potential donors, whether living or deceased, with active TB disease should not be used. ” • If negative CXR and LTBI initiate donor treatment (treatment does not need to be completed prior to transplantation)* • Deceased donors: evaluate history for previous active TB treatment – reconsider suitability of donor as clinically appropriate *consider LTBI therapy for living donor recipient Subramanian AK, Morris MI. Mycobacterium tuberculosis infections in solid organ transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2013; 13 Suppl 4: 68 -76.

2. M. 1. t 9. H. 2. d Treatment of LTBI • Active TB disease is difficult to diagnose in transplant recipients, is the cause of appreciable morbidity and mortality and is a potential public health risk • Treatment of LTBI is recommended as it significantly reduces the incidence of TB reactivation in any solid organ transplant recipient • Timing and completion of therapy prior to transplantation requires balancing risks and benefits for individual patients • Consider: current medical condition, transplant urgency, risk of progression to active TB, anticipated time til transplantation Subramanian AK, Morris MI. Mycobacterium tuberculosis infections in solid organ transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2013; 13 Suppl 4: 68 -76.

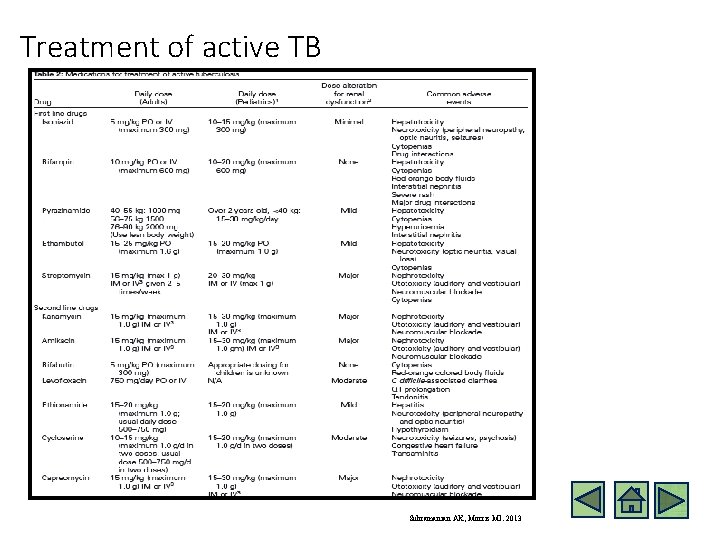
Treatment Regimens • Isoniazid (INH) for 9 months • May require dose reduction for renal insufficiency but do not change dose for dialysis • No INH interaction with common transplant medications, thus may continue INH through kidney transplant • INH may be given once daily. May also give twice weekly by directly observed therapy (DOT) • Pyridoxine (Vitamin B 6) should be administered concomitantly to reduce neurotoxicity • Dose is 25 -50 mg/kg/day • Alternate: Rifampin for 4 months • Best to complete pre-transplant due to major rifampin drug-drug interactions with common immunosuppressants (cyclosporine, tacrolimus, sirolimus, everolimus, and potentially even corticosteroids) Subramanian AK, Morris MI. Mycobacterium tuberculosis infections in solid organ transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2013; 13 Suppl 4: 68 -76.

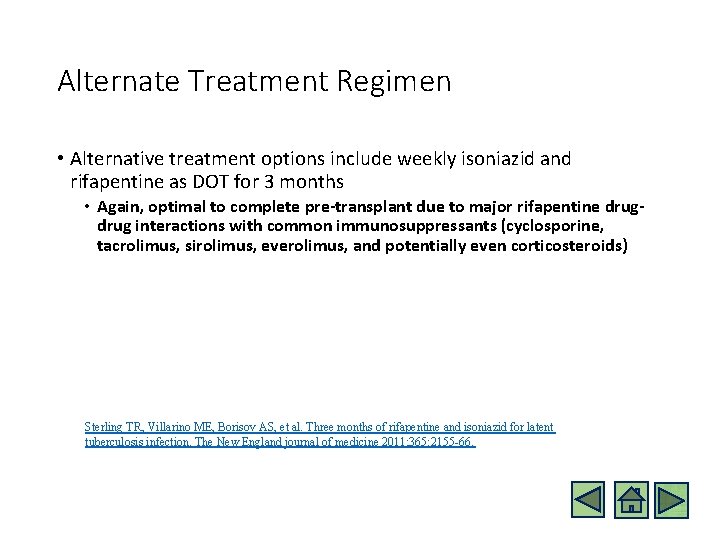
Alternate Treatment Regimen • Alternative treatment options include weekly isoniazid and rifapentine as DOT for 3 months • Again, optimal to complete pre-transplant due to major rifapentine drug interactions with common immunosuppressants (cyclosporine, tacrolimus, sirolimus, everolimus, and potentially even corticosteroids) Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. The New England journal of medicine 2011; 365: 2155 -66.

Treatment of Latent TB Subramanian AK, Morris MI. Mycobacterium tuberculosis infections in solid organ transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2013; 13 Suppl 4: 68 -76.

Treatment of active TB Subramanian AK, Morris MI. 2013

Follow up • Our patient initiated oral isoniazid– after 6 months of INH therapy, given challenges with successfully performing peritoneal dialysis (poor solute clearance) the decision was made to proceed with living donor kidney transplantation (father: donor) • Our patient completed 3 months of additional INH therapy after transplantation and is currently a healthy, successful member of his high-school baseball team.

Summary Slide • The pre-renal transplant ID evaluation includes a thorough evaluation for risks of Tuberculosis infection including travel, exposure histories. • A TST result of as little as 5 mm may be considered positive in a pre-renal transplant patient based on TB exposure, immune status and any prior treatments. • Clinicians should have a low threshold to treat LTBI (and active TB) in these patients.

Sections for Review • Case • Initial Tests • PD and HD patients are unique • LTBI screening • LTBI Treatment

This module was developed by: • Margret Bock, MD – Attending Nephrologist; Children’s Hospital Colorado, Assistant Professor of Pediatrics, University of Colorado • Eric Mc. Grath, MD – Infectious Diseases Attending, Children’s Hospital of Michigan; Associate Professor of Pediatrics, Wayne State University School of Medicine, Detroit MI Original version date: 04/18/2016 Revised on: • 09/07/2016 Part of an educational effort through the PIDS Transplant Infectious Disease working group and the AST ID Community of Practice

Evaluating This Module • PLEASE help us evaluate these modules by clicking on this link: https: //www. surveymonkey. com/r/LYMHY 6 N • For any other questions or concerns, please contact Bud Wiedermann bwiederm@childrensnational. org

Evaluating the Modules • PLEASE help us evaluate these modules by completing the pre- and post-tests included • They will help you understand retain the important material • They will help us improve future modules • For any questions or concerns, please contact Bud Wiedermann bwiederm@childrensnational. org
 How does a kidney transplant work
How does a kidney transplant work Intraosseous
Intraosseous Kidney donor transplant
Kidney donor transplant Disadvantage of kidney transplant
Disadvantage of kidney transplant Face transplant
Face transplant Face transplant
Face transplant Embryo transplants gcse biology
Embryo transplants gcse biology Dr ch madhusudhan
Dr ch madhusudhan National transplant registry malaysia
National transplant registry malaysia Slk medical abbreviation transplant
Slk medical abbreviation transplant Transplant de ficat costuri
Transplant de ficat costuri Oncogens
Oncogens Next
Next Bone marrow transplant diet
Bone marrow transplant diet Pichere pasare
Pichere pasare Eliza ann grier
Eliza ann grier Leukemoid reaction
Leukemoid reaction Neograft hair transplant in miami
Neograft hair transplant in miami Ppd
Ppd Pejabat daerah kota kinabalu
Pejabat daerah kota kinabalu Linfohematogena
Linfohematogena Ppd setiu
Ppd setiu Mantoux
Mantoux Laporan pbd
Laporan pbd Ppd
Ppd Ppd portfolio
Ppd portfolio Mechanism of action of dapsone
Mechanism of action of dapsone Incoterm ppd
Incoterm ppd Pengertian ppd
Pengertian ppd Ppd
Ppd Interpretação ppd
Interpretação ppd