The Good Fight Provider Status Drug Shortages and
























- Slides: 25

The Good Fight: Provider Status, Drug Shortages, and Drug Pricing Jillanne Schulte Wall, J. D. Director, Federal Regulatory Affairs

Key Issues • Provider Status • Drug Shortages • Drug Pricing

Provider Status • ASHP, along with PAPCC, seeking to roll provider status into a larger health care bill • Congress turning its attention to midterm elections in November – Opportunities for additional healthcare legislation limited for the remainder of 2018 – Anticipate movement on opioid-focused legislation • Given the narrower focus on opioids, PAPCC focused attention on finding a way to incorporate pharmacists and their role in addressing the epidemic

Provider Status • Intent of the provider status effort was always to enhance our members’ ability to provide care – Goal of recognition as part of interprofessional care team still stands – This can be achieved through multiple avenues • ASHP supports state-level efforts to expand scope of practice in addition to working at the federal level to include pharmacists in Medicare

Drug Shortages • Drug Shortages – – – – Small-volume parenteral solutions (SVP) Sodium bicarbonate Epinephrine Morphine Hydromorphone Sterile water Amino acids (used for IV nutrition)

Drug Shortages: Practice Advancement Efforts • 20+ years of leadership • Drug Shortages Resource Center – Ongoing partnership with University of Utah Drug Information Service • Conservation strategies and best practices • Led a roundtable discussion on shortages that included clinician groups, the FDA, and HHS – Recommendations for long-term policies – Highlighted need for greater transparency, redundancy in manufacturing – Exploring solutions to avoid or mitigate critical shortages

Drug Shortages: Hill Strategy • Spearheaded letter to Hill – Need to reopen dialog on shortages – Outlined critical questions • Congressional meetings – Staff and members of House Energy & Commerce and Senate Health, Education, Labor, & Pensions committees – Individual key offices • Congressional sign on letter to FDA on shortages – ASHP supported and worked with key members on letter

IV Opioid Shortages • ASHP has been working with DEA to ensure that active product ingredient is getting to manufacturers who can fill in the gaps left by Pfizer’s reduced capacity • February 27, 2018: ASHP spearheaded a joint letter to DEA asking them to use their discretionary authority to approve revisions of API quotas – AHA, ISMP, ASCO, and ASA also signed the letter • May 4, 2018: ASHP spearheaded a joint comment letter to DEA requesting that shortages be included as a factor in any changes to quotas and that federal agencies provide DEA with information related to current and anticipated shortages – Same signatories as the Feb. 27 th letter, with the addition of AHIP

Shortages and Global Trade Policy • Potential disruption of supply channels due to tariffs on active product ingredient (API) • ASHP submitted a letter to the United States Trade Representative requesting that no tariffs be applied to API without a full impact review by FDA • In addition to potentially exacerbating shortages, tariffs on API also carry the risk of spiking drug prices

Game Change: Hospitals Fight Back • Civica RX – Non-profit formed by hospitals to ensure a steady supply of the generic drugs – Starting with 14 hospital-administered drugs, which have not yet been announced • Founding members include: – – – Catholic Health Initiatives HCA Healthcare Intermountain Healthcare Mayo Clinic SSM Health Trinity Health

Drug pricing • Off patent drugs with no generic equivalent • Misuse of REMS to manipulate the market • Lack of competition in generic market

Campaign for Sustainable Rx Pricing • Non-partisan coalition of organizations, including AHA, AARP, AHIP, ASHP, Greater NY Hospital Association, and Walmart • Members span consumer, payer, and provider spectrum • Exploring market-based solutions, not price controls • Endorsing legislation aimed at addressing the high cost of drugs

Drug pricing legislation • Over 20 unique bills introduced in House and Senate to date • Bills fall under following categories: § Importation § Direct Federal government negotiation § Fostering competition

Congress takes note • H. R. 2212/S. 974 – “Creating and Restoring Equal Access To Equivalent Samples Act of 2017” (CREATES Act) – Reduces ability of brand drug to manipulate market – ASHP supports bill • S. 124, the “Preserve Access to Affordable Generics Act” of 2017, and S. 297, the “Increasing Competition in Pharmaceuticals Act” of 2017. – Increases competition by prohibiting “pay-to-delay” tactics or expediting reviews of a generic drug where there are currently no generic alternatives – ASHP supports these bills

HHS Drug Pricing Plan • On May 11, 2018, HHS released the Administration’s plan to combat high drug prices: – Proposals are in outline form – ASHP support will be contingent on the final policy and implementation details for each proposal • Generally, we will support proposals that align with our policies around increased transparency and improved patient access and reduced patient out-of-pocket costs – We are concerned about the 340 B proposals and some of the suggested changes to Medicare and Medicaid • The Blueprint does not touch on importation, but ASHP remains opposed to importation except to mitigate drug shortages

Promising Drug Pricing Plan Proposals Removal of the Part D gag clause Allowing more substitution in Part D Reducing patent gaming Increasing transparency and improving communication with Part D beneficiaries • Improving biosimilar access • •

Areas of Concern in the Drug Pricing Plan Merging Part B drugs into Part D Site neutrality in Part B payment Changes to Medicare and Medicaid Rebates Demonstration Projects, including value-based payment models that include major changes to payment • 340 B Changes • •

340 B Program • July and October 2017: House Energy and Commerce Committee holds hearing on 340 B – Concerns expressed over HRSA regulatory authority – How covered entities use the program – Lack of transparency • January 2018: House Energy and Commerce Committee releases report on 340 B, specifically calling for changes to the program • Senate HELP held 3 hearings

340 B Program • Changes suggested in the report: – – – HRSA regulatory authority & resources Independent audits Reduce duplicate discounts with Medicaid Promote transparency in the program Define charity care Reassess DSH as appropriate measure for program eligibility

340 B Program • March 2018: Senate Health, Education, Labor and Pensions (HELP) Committee holds hearing on 340 B – ASHP called to testify – Outlined how members interact with program – Highlight efforts to remain compliant

340 B Program • Legislative efforts underway, many bills in Congress, diverse range: – – Undue CMS cuts Moratorium on DSH child sites Reporting and transparency Define charity care, patient • Potential for more hearings?

340 B Program • Questions for covered entities: – Is no change to 340 B a long-term winning strategy? – If not, what policies can we support: • HRSA authority, resources • Transparency and reporting on how savings are utilized • Broad definitions of charity care and patient definition (HRSA could do that) • Are CMS cuts negotiating tool? • Any changes to the program should include efforts to tighten manufacturer oversight • ASHP gathering member input on questions above • November may change House composition but challenges to program will remain

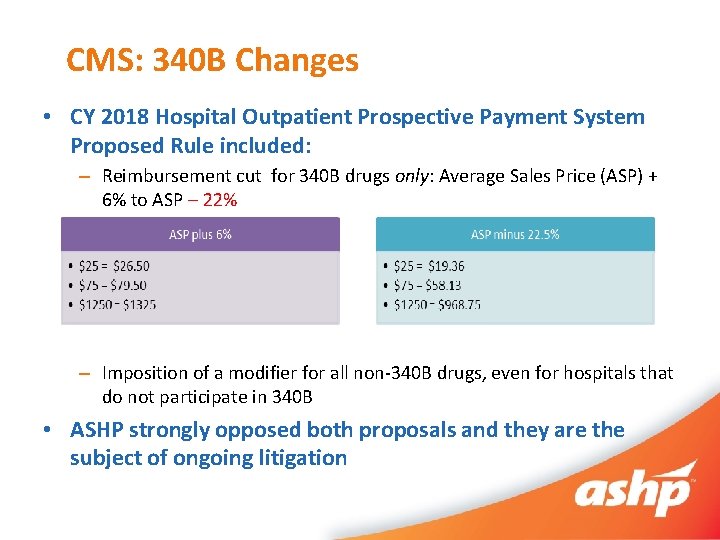
CMS: 340 B Changes • CY 2018 Hospital Outpatient Prospective Payment System Proposed Rule included: – Reimbursement cut for 340 B drugs only: Average Sales Price (ASP) + 6% to ASP – 22% – Imposition of a modifier for all non-340 B drugs, even for hospitals that do not participate in 340 B • ASHP strongly opposed both proposals and they are the subject of ongoing litigation

Other 340 B Changes • On May 5, 2018, HRSA announced that it was pulling back the Civil Monetary Penalties rule, which would have increased oversight of manufacturers participating in the 340 B program – The rule had already been delayed a number of times by the Trump Administration – AHA and other groups are suing CMS over the rule • The HHS Drug Pricing Blueprint, released in May, targeted 340 B as a cause of high drug prices – No concrete proposals were made, but there was discussion of patient definition, savings tracking, and HRSA authority

Questions?
 Inventory model with planned shortages
Inventory model with planned shortages Supply chain shortages are
Supply chain shortages are Difference between scarcity and shortage in economics
Difference between scarcity and shortage in economics An example of crude drug adulterated with exhausted drug
An example of crude drug adulterated with exhausted drug 2 timothy 4:7-8 meaning
2 timothy 4:7-8 meaning Good thoughts, good words, good deeds
Good thoughts, good words, good deeds Hi, good evening
Hi, good evening Good afternon animado
Good afternon animado You are good when there's nothing good in me
You are good when there's nothing good in me Buenas tardes
Buenas tardes Function of pituitary gland in points
Function of pituitary gland in points Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Chụp tư thế worms-breton
Chụp tư thế worms-breton Hát lên người ơi
Hát lên người ơi Môn thể thao bắt đầu bằng từ chạy
Môn thể thao bắt đầu bằng từ chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 độ dài liên kết
độ dài liên kết

















































