Safety and Financial Impact of Drug Shortages on




















































- Slides: 61

Safety and Financial Impact of Drug Shortages on Hospitals and The Release of the 2011 ISMP Medication Safety Self Assessment® Allen J. Vaida, Pharm. D. Executive Vice President Institute for Safe Medication Practices avaida@ismp. org

Speakers • Allen J. Vaida, Pharm. D Institute for Safe Medication Practices • Dwight Kloth, Pharm. D, Director of Pharmacy Fox Chase Cancer Center, Philadelphia, PA • Joseph M. Hill Director, Federal Legislative Affairs American Society of Health-System Pharmacists • Roslyne D. W. Schulman Director, Policy Development American Hospital Association

Drug Shortages • FDA Presents at ASHP midyear - 2000 • First ISMP survey - 2001 • ASHP-University of Utah partnership – 2001 • Stakeholder meeting 2002 • 2008 – 2011 crisis mode

Drug Shortages: a Serious and Widespread Problem

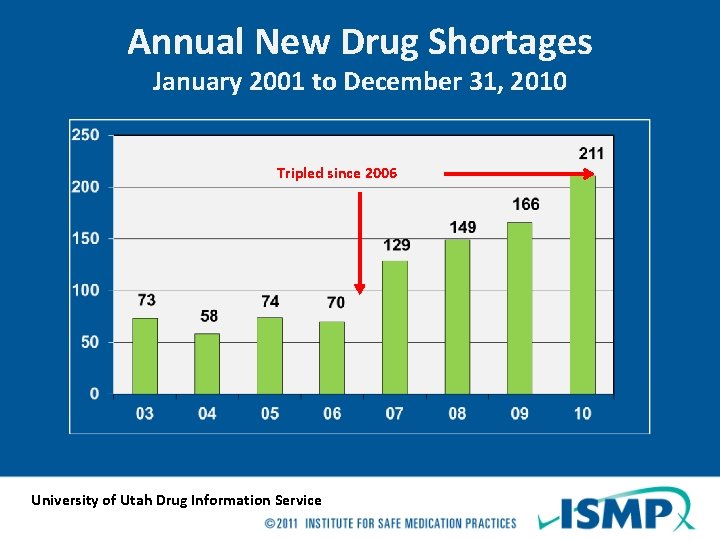
Annual New Drug Shortages January 2001 to December 31, 2010 Tripled since 2006 University of Utah Drug Information Service

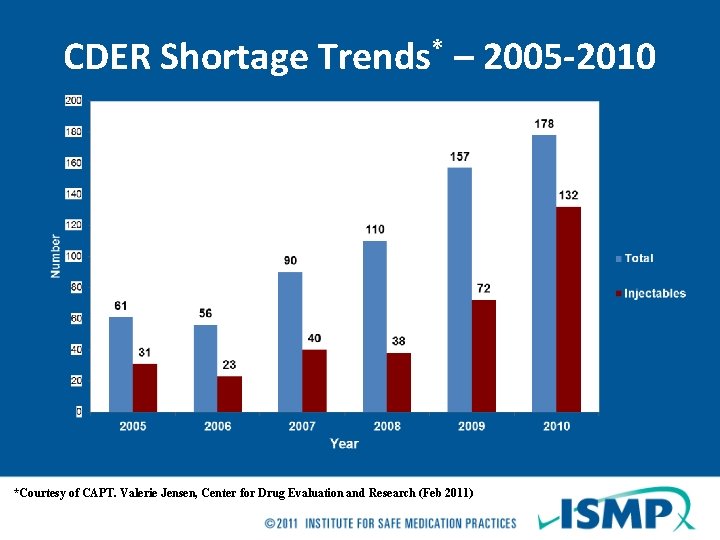
CDER Shortage Trends* – 2005 -2010 *Courtesy of CAPT. Valerie Jensen, Center for Drug Evaluation and Research (Feb 2011)

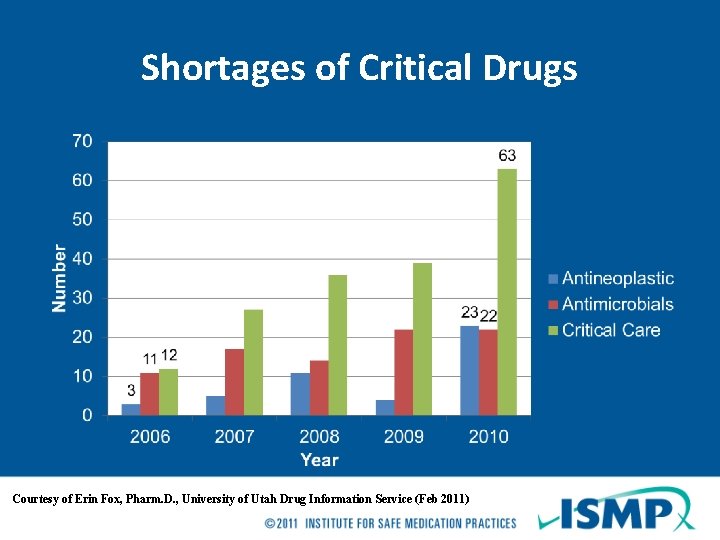
Shortages of Critical Drugs Courtesy of Erin Fox, Pharm. D. , University of Utah Drug Information Service (Feb 2011)

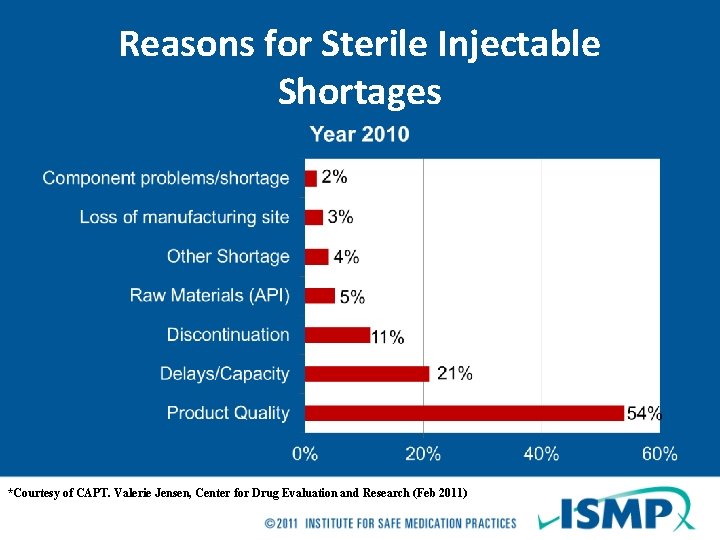
Reasons for Sterile Injectable Shortages *Courtesy of CAPT. Valerie Jensen, Center for Drug Evaluation and Research (Feb 2011)

2010 ISMP 2 nd Survey on Drug Shortages ISMP Med Saf Alert. 2010; 15(19): 1 -3

What We Heard from Respondents • Clinical effects – Compromise or delay in medical treatment/procedures – Medication errors and adverse patient outcomes • Financial effects – Higher alternative acquisition costs – Reallocation and/or addition of staff – Management of adverse events • Emotional effects – Strained professional relationships

Near Misses, Errors, Adverse Outcomes

Most Problematic Shortages • • Propofol Succinylcholine Heparin Naloxone Bumetanide and Furosemide Emergency syringes Chemotherapy medications

Top Difficulties Reported No advanced warning and suggested alternatives No information about cause of shortage No information about duration of shortage Difficulty obtaining suitable alternatives Substantial resources educating practitioners on the use of alternatives • Possible loss of prior safety safeguards put in place • • •

Issues Identified with Alternative Medications • Enhanced risk of errors and/or adverse outcomes - difficulty remembering alternative drugs and how to safety prescribe, dispense, administer • Ethical dilemma with rationing - who gets the optimal drug – especially with oncology

Recent Survey Results from Premier (3/28) Premier reported that -- surveyed 311 pharmacy experts at hospitals and other facilities, such as surgery centers and long-term care facilities, about shortages during a six-month period in 2010. " Investigators eventually discovered that "89% had experienced shortages that may have caused a medication safety issue or error in patient care. "

ASHP Drug Shortages Summit November 5, 2010 • Goals – define the scope, causes, and potential patient harm from drug shortages – discuss potential changes in public policy and stakeholder practices • Partner Organizations – American Society of Anesthesiologists – American Society of Clinical Oncology – Institute for Safe Medication Practices

Drug Shortages Summit • 50+ participants, including representatives from: – – – health professional organizations pharmaceutical manufacturers supply chain entities FDA CDC (Centers for Disease Control and Prevention)

Summit Outcomes • 21 recommendations – Increase sharing of information among stakeholders and – Remove barriers faced by the FDA and drug manufacturers • Areas of focus – – Regulatory/Legislative (13) Raw Materials/Manufacturing (4) Business/Market (2) Distribution (2) Available at: www. ashp. org/drugshortages/summitreport

Business and Market Factors • Lack of transparency or communication about actual or possible product shortages • Lack of business incentives to enter a specific product market • Unpredictable changes in product demand • Reallocation of production lines • Consolidation of companies

Raw Materials and Manufacturing Factors • 80% of raw materials used come from outside the United States • Disruption to acquisition can be due: – Political instability/Government interference – Natural disasters – Contamination during production, storage or transport • Problematic if single source Active product ingredients (API) or raw materials multiple manufacturers affected

Raw Materials and Manufacturing Factors • Major manufacturing difficulties identified: – Inability to comply with current good manufacturing practices (c. GMP) and/or voluntary recall – Limited number of production lines � Increase production of one product results in shortage of another – Complexity of manufacturing sterile injections – Loss of experienced personal due to business decisions – Change in product formulation

Distribution Factors • Inventory practices by healthcare facilities and supply chain entities • Little or no inventory cushion to address short-term shortages or excess inventory due to distribution systems • Variability in inventory procurement capabilities between small and large healthcare facilities • Grey market

Regulatory and Legislative Factors • Limited FDA resources for timely inspection of manufacturing sites and review of NDA/ANDA • Lack of FDA authority to: – Require notification from manufacturers of anticipated market withdrawal – Enforce notification requirements for medically necessary products

Drug Shortages Summit: Next Steps • • Workgroups begin meetings 1 st week of April Prioritizing and work plans – Not all recommendations will be implemented • Advocacy – Congressional meetings – Media outreach (trade and lay press)

Impact of Drug Shortages 2011 Lessons from the Front Lines Dwight D. Kloth, Pharm. D. , FCCP, BCOP Fox Chase Cancer Center

Impact on Hospital Pharmacy Departments and other Clinical Staff • Impact on the Pharmaceutical Purchaser • Value of the Pharmaceutical Purchaser • Impact on Inventory Turn Ratios and “Just in Time Ordering” • Impact on Cost of Product • Impact on other pharmacy personnel – – – Assistant/Associate Director of Pharmacy Clinical Coordinator Pharmacists Technicians

Impact on Hospital Pharmacy Departments and other Clinical Staff • Impact on the P&T Committee • Impact on medical, nursing and pharmacy staff • Need for teamwork within the institution, vital need to avoid “finger pointing” • Increased risk of medication error • Role of P&T Committee Newsletters and e-mails (advance warning notice weeks in advance of a possible out of stock condition) • Role of the ISMP Medication Safety Alert September 23, 2010

Impact on Hospital Pharmacy Departments and other Clinical Staff • Impact on chemotherapy order templates or CPOE order sets (revisions if required to switch any medications, pre-meds, cytotoxics, etc) • Increased risk of medication error – inpatient – ambulatory infusion room – operating room • Gray Market (Black Market)- price gouging • Premier Healthcare Alliance analysis (March, 2011) $ 200 million annual cost to US hospitals

Recent Examples Significantly Impacting Patient Care • Propofol in summer and fall of 2009 – Hospira recall – Teva recall (& subsequent exit from market) • Baxter heparin recall – tainted Chinese heparin supplier 2009 • Morphine vs. hydromorphone (e. g. , errors) • Etoposide • Neuromuscular Blocking Agents

Current Severe Examples in Acute Care • Potassium Phosphate – “Dear Healthcare Professional” letter 3 -28 -2011 – filter all lot #s of the product in Pharmacy – In-line 0. 22 micron filter, impact on Nursing and cost of IV tubing – only one manufacturer in the USA at the moment- no alternatives • • MVI for TPN- being rationed by CAPS Calcium Gluconate injection- rationing begun Norepinephrine Lorazepam injection

Current Severe Examples in Oncology • Cytarabine • Thiotepa – Request from a major cancer center to trade with FCCC; FCCC did not have enough to even consider • Bleomycin • Doxorubicin • Leucovorin (generic racemic leucovorin) – Request received from a 2 nd opinion patient for FCCC to give drug supply to the patient’s hospital 70 miles away

Drug Shortages: Update on ASHP Actions and Advocacy April 7 , 2011 Joe Hill Director of Legislative Affairs American Society of Health-System Pharmacists

Drug Shortages Summit: Next Steps Workgroups begin meetings 1 st week of April Prioritizing and work plans – Not all recommendations will be implemented Advocacy – Congressional meetings – Media outreach (trade and lay press)

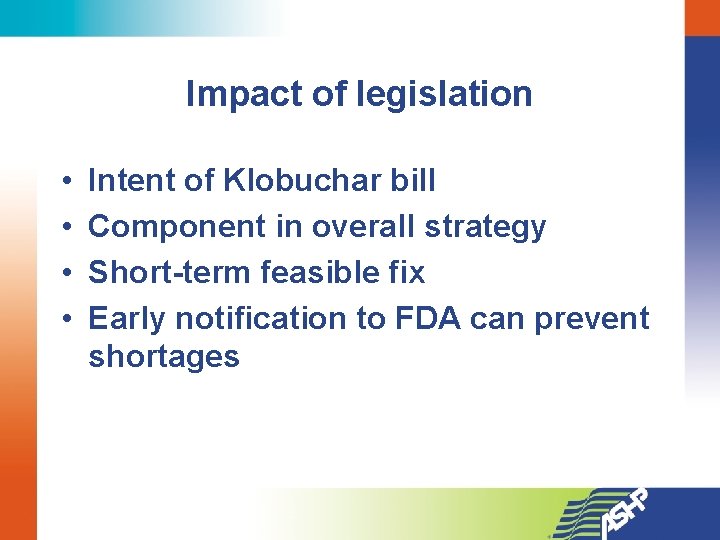
Impact of legislation • • Intent of Klobuchar bill Component in overall strategy Short-term feasible fix Early notification to FDA can prevent shortages

Drug Shortages: Congressional Action • ASHP met with Senator Amy Klobuchar (D-Minn) late last year • Expressed their intent to introduce legislation • Aggressive timeline made it impossible to address everything • ASHP prioritized, identified reporting of manufacturing problems as the place to start

Preserving Access to Life-Saving Medications Act S. 296 • New bill: Senator Klobuchar and Senator Casey • Manufacturers to notify FDA of any interruption in production of any product 6 months in advance, or ASAP • FDA to have an enforcement mechanism— fines for not complying • FDA to develop criteria for “vulnerable” drugs • Firms to develop continuity of supply plans for those drugs [in collaboration with FDA]

Shortage Bill, continued • FDA would track vulnerable drugs – Enhanced focus and monitoring by FDA drug availability – Prioritization of FDA approval procedures – Work with firms to resolve problems • No public notification of drugs “vulnerable” to shortage

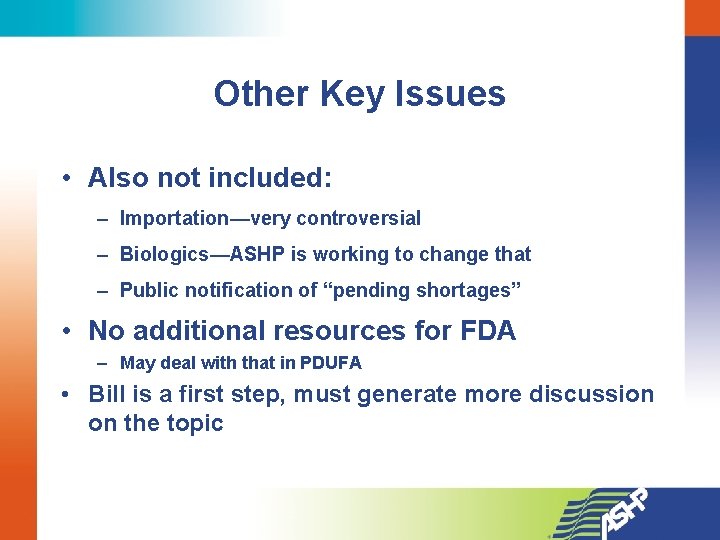
Other Key Issues • Also not included: – Importation—very controversial – Biologics—ASHP is working to change that – Public notification of “pending shortages” • No additional resources for FDA – May deal with that in PDUFA • Bill is a first step, must generate more discussion on the topic

What is the Plan? • Bill introduced February 7 • Cosponsors include Senators Blumenthal (Conn), Cardin (MD) • Looking at a dual track approach—support 296 (i. e. , reporting requirements, vulnerable products) and pursue other issues/resources through PDUFA reauthorization in 2012 • Continuing to work with stakeholders, Congress

No easy fix……. Solution will require dedicated short- and long-term effort from all stakeholders

What can YOU do? Share information and solutions with colleagues and clients Support drug shortages advocacy initiatives Contact your legislators; invite them to your hospital Tell your stories Emphasize the risks to public health and patient safety CONTINUE TO REPORT DRUG SHORTAGES

2011 ISMP Medication Safety Self Assessment® • Funded by the Commonwealth Fund • Available online • The self assessment will help hospitals to: – Evaluate their safety practices – Identify opportunities for improvement – Compare their experiences over time with those of similar organizations

Background • First assessment in 2000 followed the IOM report ‘To Err is Human’ and was the first nationwide look at medication safe practices • All assessments are in cooperation with the American Hospital Association, the Health Research and Education Trust and funded by the Commonwealth Fund • Many prior safety items are incorporated in the Joint Commission safety goals and the National Quality Forum best practice recommendations

Content • Ten elements (domains) that most significantly influence safe medication use • Twenty core distinguishing characteristics of a safe medication system • Representative self-assessment characteristics

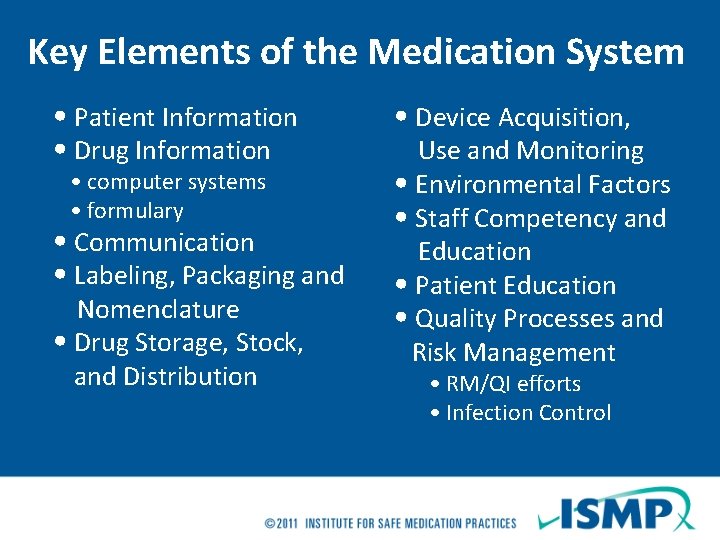
Key Elements of the Medication System • Patient Information • Drug Information • computer systems • formulary • Communication • Labeling, Packaging and Nomenclature • Drug Storage, Stock, and Distribution • Device Acquisition, Use and Monitoring • Environmental Factors • Staff Competency and Education • Patient Education • Quality Processes and Risk Management • RM/QI efforts • Infection Control

Prior Assessments • 1434 hospitals submitted data in 2000 and 1623 hospitals submitted data in 2004 • States, individual hospitals, health systems and collaboratives have continued to re-assess themselves since 2004

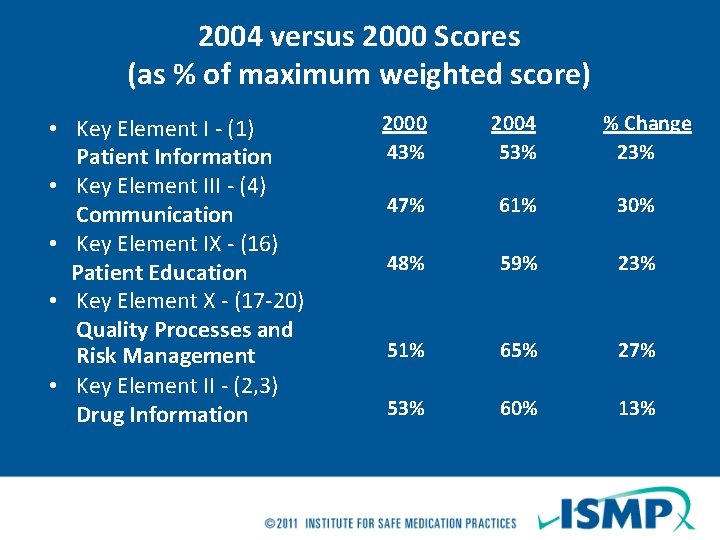
2004 versus 2000 Scores (as % of maximum weighted score) • Key Element I - (1) Patient Information • Key Element III - (4) Communication • Key Element IX - (16) Patient Education • Key Element X - (17 -20) Quality Processes and Risk Management • Key Element II - (2, 3) Drug Information 2000 2004 43% 53% % Change 23% 47% 61% 30% 48% 59% 23% 51% 65% 27% 53% 60% 13%

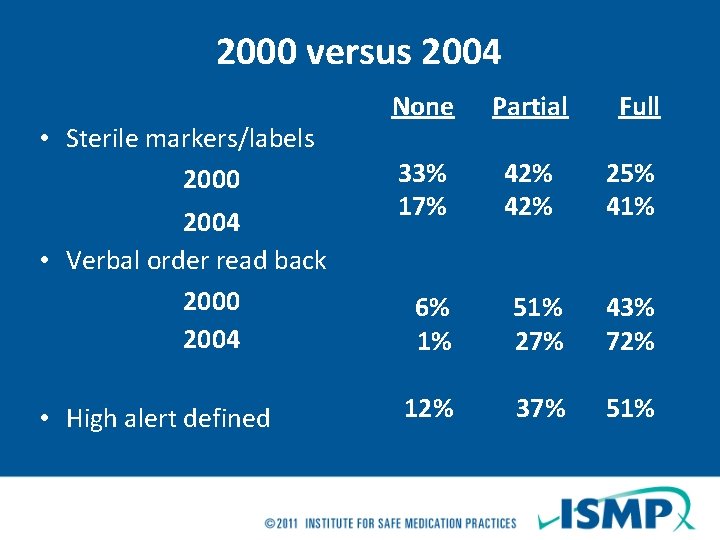
2000 versus 2004 • Sterile markers/labels 2000 2004 • Verbal order read back 2000 2004 • High alert defined None Partial Full 33% 17% 6% 1% 12% 42% 25% 41% 51% 27% 43% 72% 37% 51%

Items for the 2011 Self Assessment • Review of ISMP error reports and ISMP publications since 2004 • Review of the safety literature including other sources of reports on adverse drug events • National Advisory Panel of a multidisciplinary group of practitioners

New Items for 2011 • Expanded safe use of technology • Use of metrics from technology to help measure improvement • Processes for the handling of drug shortages, maintenance of technology, and the safe environment for medication use • Elements of accountability within a Just Culture

Patient and Drug Information • The hospital utilizes a surgical safety checklist (i. e. , the World Health Organization [WHO] Surgical Safety Checklist or an adaptation) prior to surgical procedures to verify patient identity, allergies, and preoperative antibiotics (when required). • Equianalgesic dosing charts for oral, parenteral, and transdermal (e. g. , fenta. NYL patches) opioids have been established and are easily accessible to all practitioners when prescribing, dispensing, and administering opioids.

Technology/Devices • General infusion pumps with SMART PUMP TECHNOLOGY with full functionality employed to intercept and prevent wrong dose/wrong infusion rate errors due to misprogramming the pump… are in use in all hospital areas (including the ED, pediatrics, oncology, operating room). • The administration set used for epidural infusion pumps does not contain any access ports (Y connectors), can be distinguished from all other administration sets and medical tubing (e. g. , a yellow stripe running the length of the tubing), and is not used for anything other than epidural infusions.

Drug Stanadization • IV solutions that are unavailable commercially are prepared in the pharmacy unless needed in emergent lifesaving situations. • Pharmacy fills all elastomeric pumps and prepares all IV solutions and irrigations needed in the operating room or procedural areas (including interventional radiology, cardiac catheterization areas), unless needed in emergent lifesaving situations.

Infection Control • Pen devices that contain multiple doses of medication (e. g. , insulin pens) are dispensed for individual patients and are never used as unit stock for multiple patients, even if the needle is changed between patients or the medication is withdrawn from the pen cartridge with a sterile syringe. • In patient care areas, multiple-dose vials are not used for saline and heparin flush solutions, or local anesthetics. Exception: Local anesthetics used in the operating room that are restricted to a single patient procedure.

Multidisciplinary Team • CEO, COO, or Senior level Vice President in charge of Quality and Safety • Chief Medical Officer • Nurse Executive • Director of Pharmacy • Chief Information Officer • Front line managers

Completing the Assessment • Secure and anonymous password issued • Ability to download a pdf file and save information between team meetings • Ability to complete on-line and save information between team meetings • Immediate scoring of the assessment once the data is submitted • Comparisons to similar organizations when the assessment data entry period is complete

Choice Selections • A. There has been no activity to implement this item. • B. This item has been formally discussed and considered, but it has not been implemented. • C. This item has been partially implemented in some or all areas of the organization. • D. This item is fully implemented in some areas of the organization. • E. This item is fully implemented throughout the organization.

Individual Items Weighted According to Impact on System • Scale of 4 to 16 with higher weights on items that: Ø Target the system not workforce Ø Simplify complex processes Ø Solve several error-prone problems Ø Do not rely heavily on human memory or vigilance

Timeline and Promotion • Data submission through the end of August, 2011 • Promotion by AHA, HRET, state hospital associations, endorsers, supporters, and leadership in hospitals • Commitments from national collaboratives

Endorsers • • • American Association of Colleges of Nursing American Hospital Association American Nurses Association American Organization of Nurse Executives American Pharmacists Association American Society for Healthcare Risk Management American Society of Health-System Pharmacists American Society of Medication Safety Officers Amerinet Anesthesia Patient Safety Foundation • • • • Association of American Medical Colleges Child Health Corporation of America Federation of American Hospitals Health Care Improvement Foundation Health Research and Educational Trust Healthcare Information and Management Systems Society Institute for Healthcare Improvement The Joint Commission Med. Assets National Patient Safety Foundation Pennsylvania Patient Safety Authority Premier University Health. System Consortium VHA

Questions
 Inventory model with planned shortages
Inventory model with planned shortages Supply chain shortages are
Supply chain shortages are Definition of scarcity in economics
Definition of scarcity in economics Deliberate adulteration examples
Deliberate adulteration examples Drug and alcohol safety training
Drug and alcohol safety training The required safety inventory
The required safety inventory Non-financial methods of motivation
Non-financial methods of motivation Four shades function for the encs
Four shades function for the encs Qbs safety care
Qbs safety care Process safety vs personal safety
Process safety vs personal safety Ind safety report
Ind safety report 00101-15 basic safety
00101-15 basic safety Basic safety construction site safety orientation
Basic safety construction site safety orientation Hillingdon arch
Hillingdon arch Drugs that alter moods, thoughts, and sense perceptions
Drugs that alter moods, thoughts, and sense perceptions Dono in pharmacognosy
Dono in pharmacognosy Active pharmaceutical ingredient sourcing
Active pharmaceutical ingredient sourcing Center for drug evaluation and research
Center for drug evaluation and research Career opportunities in biotechnology and drug development
Career opportunities in biotechnology and drug development Tadra is an acronym for
Tadra is an acronym for Regulatory agencies
Regulatory agencies Ward basket system
Ward basket system Drug metabolism and pharmacokinetics
Drug metabolism and pharmacokinetics 12 core functions of counseling
12 core functions of counseling Drug and alcohol jeopardy
Drug and alcohol jeopardy Punch method of capsule filling
Punch method of capsule filling Rate limiting steps in drug absorption
Rate limiting steps in drug absorption Dosage forms and drug delivery systems
Dosage forms and drug delivery systems Forensic pharmacy
Forensic pharmacy Drug and alcohol information system (daisy)
Drug and alcohol information system (daisy) Natural selection and drug resistance
Natural selection and drug resistance Natural selection and drug resistance
Natural selection and drug resistance Centre county drug and alcohol
Centre county drug and alcohol Parenteral dosage form example
Parenteral dosage form example Whats a muckraker
Whats a muckraker Abbreviated new drug application
Abbreviated new drug application Parenteral dosage form examples
Parenteral dosage form examples Investigating and making a case for drug diversion
Investigating and making a case for drug diversion A depressant drug drivers ed
A depressant drug drivers ed Jordan food and drug administration
Jordan food and drug administration Drug identification and toxicology
Drug identification and toxicology Projectile apparatus with impact board and launching ramp
Projectile apparatus with impact board and launching ramp Impact of hospitalization on family
Impact of hospitalization on family Top ten risk item tracking
Top ten risk item tracking Impact pressure chamber and lines
Impact pressure chamber and lines Impact of computers in today's society
Impact of computers in today's society What you mean by renaissance
What you mean by renaissance Pursuit of sovereignty and impact of partition essays
Pursuit of sovereignty and impact of partition essays Digital communications and networks impact factor
Digital communications and networks impact factor Success pros
Success pros Materi kuliah manajemen resiko
Materi kuliah manajemen resiko Lesson 5 the slave trade and its impact on africa
Lesson 5 the slave trade and its impact on africa What is the scope and economic impact of agribusiness
What is the scope and economic impact of agribusiness What is the scope and economic impact of agribusiness?
What is the scope and economic impact of agribusiness? What is the scope and economic impact of agribusiness?
What is the scope and economic impact of agribusiness? What is the scope and economic impact of agribusiness
What is the scope and economic impact of agribusiness Research impact and researcher identity
Research impact and researcher identity Look alike drug list
Look alike drug list Ideal drug examples
Ideal drug examples Drug calculation formula
Drug calculation formula In the u.s., the most heavily abused drug(s) is/are:
In the u.s., the most heavily abused drug(s) is/are: Drug cabinet in the brain
Drug cabinet in the brain