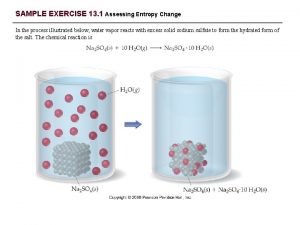
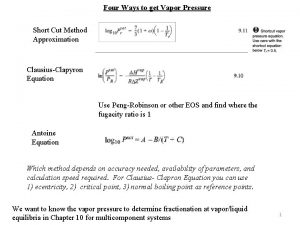
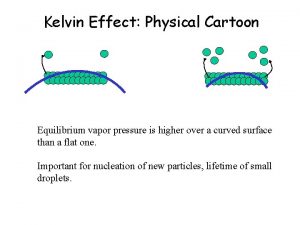
The effect of applied pressure on vapor pressure









- Slides: 20

The effect of applied pressure on vapor pressure

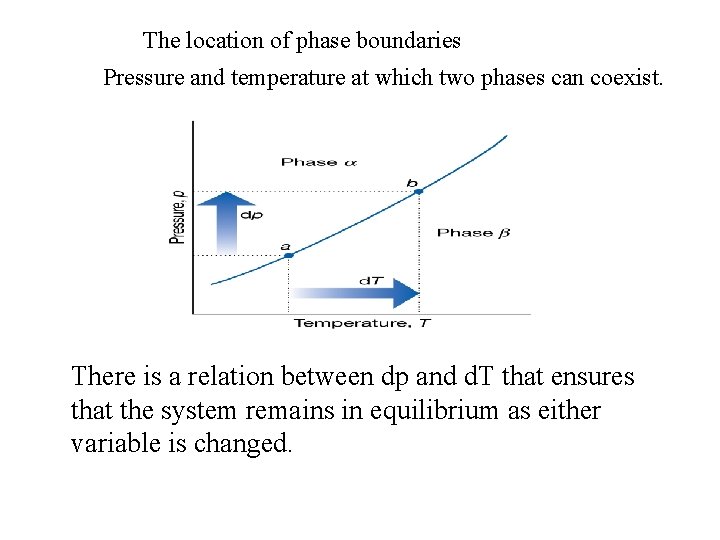
The location of phase boundaries Pressure and temperature at which two phases can coexist. There is a relation between dp and d. T that ensures that the system remains in equilibrium as either variable is changed.

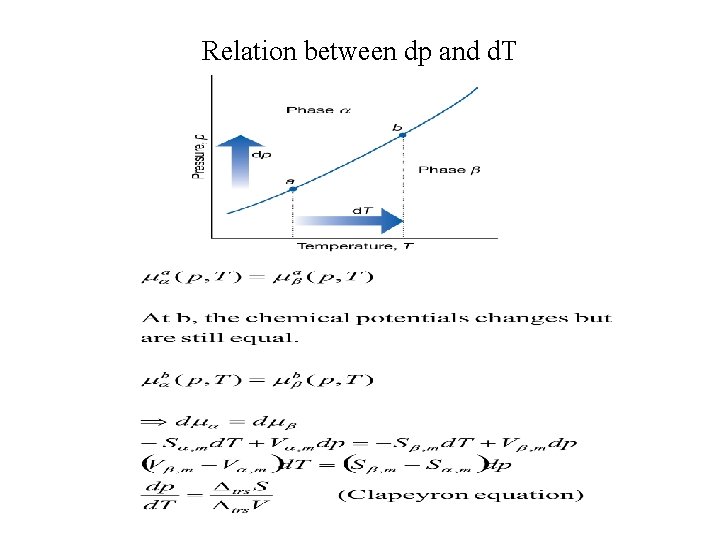
Relation between dp and d. T

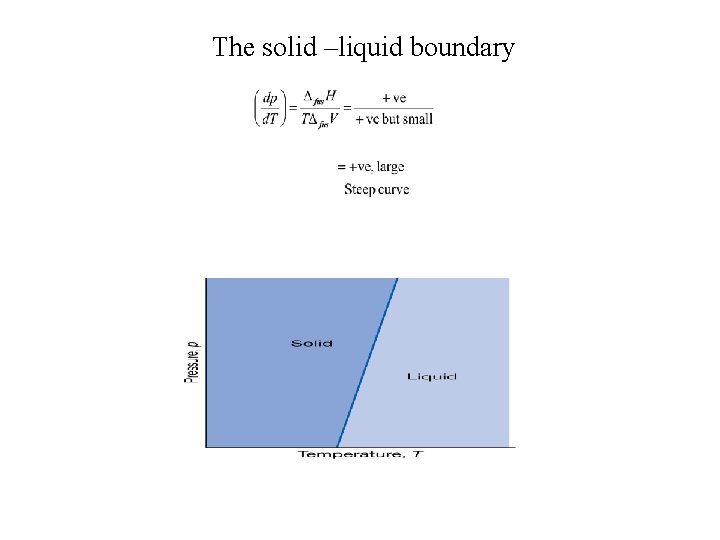
The solid –liquid boundary

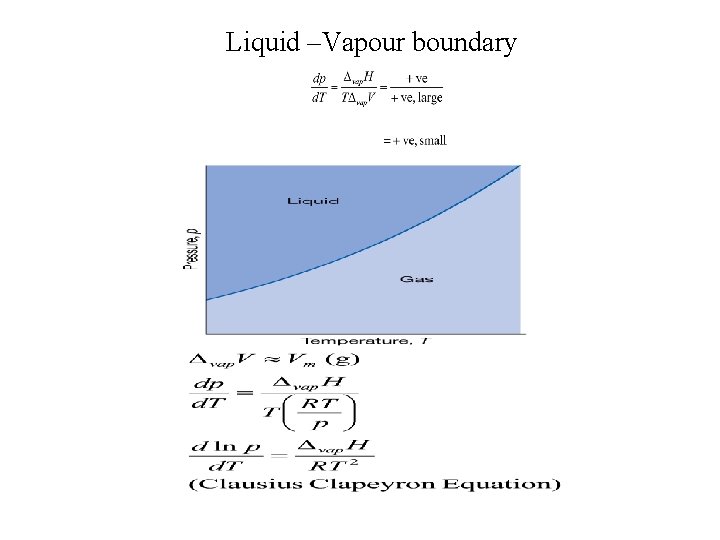
Liquid –Vapour boundary

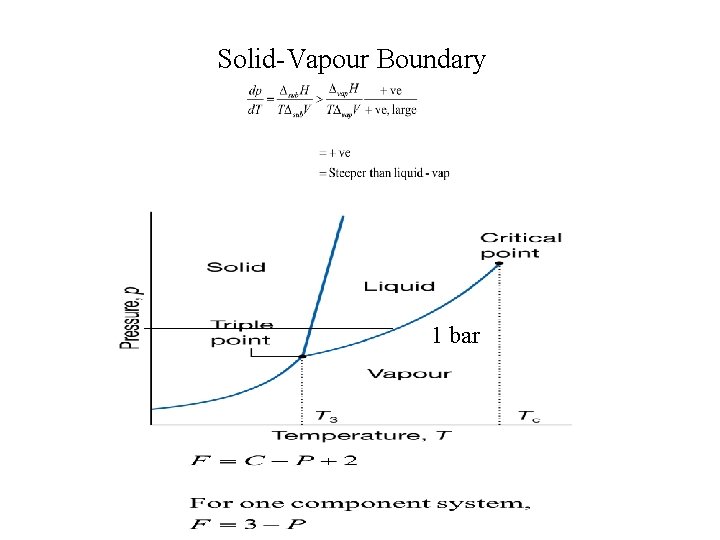
Solid-Vapour Boundary 1 bar

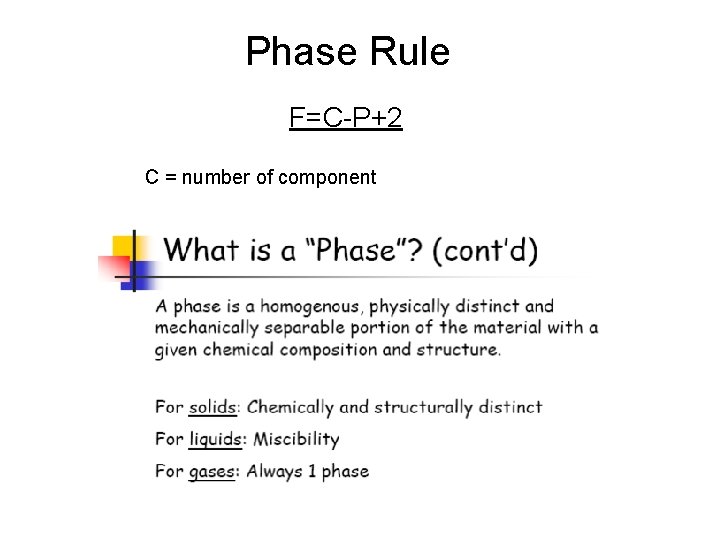
Phase Rule F=C-P+2 C = number of component

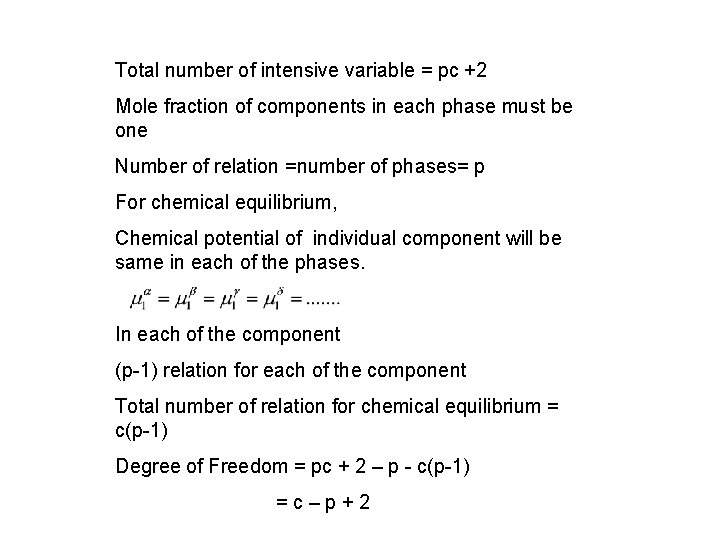
Total number of intensive variable = pc +2 Mole fraction of components in each phase must be one Number of relation =number of phases= p For chemical equilibrium, Chemical potential of individual component will be same in each of the phases. In each of the component (p-1) relation for each of the component Total number of relation for chemical equilibrium = c(p-1) Degree of Freedom = pc + 2 – p - c(p-1) =c–p+2


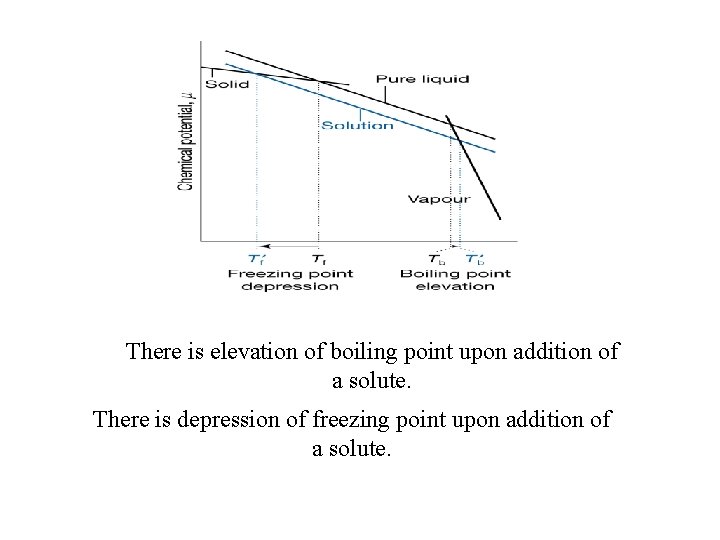
What happens to boiling point or freezing point of a liquid in the presence of a solute? What happens to chemical potential of a liquid in the presence of solute?

There is elevation of boiling point upon addition of a solute. There is depression of freezing point upon addition of a solute.

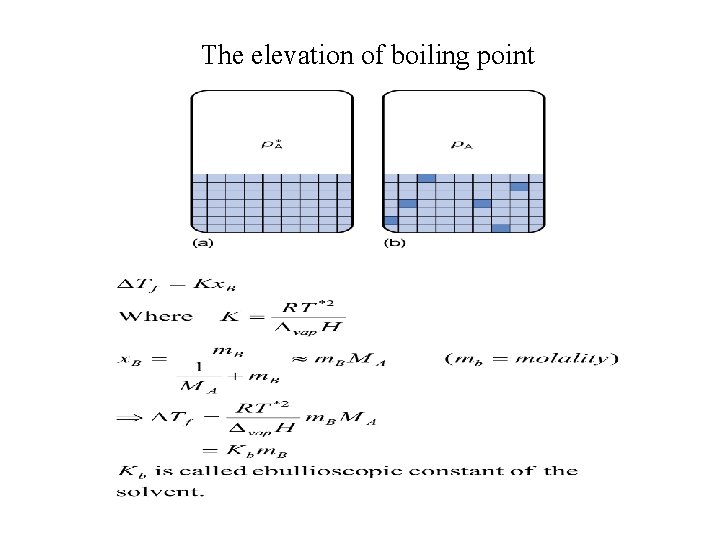
The elevation of boiling point



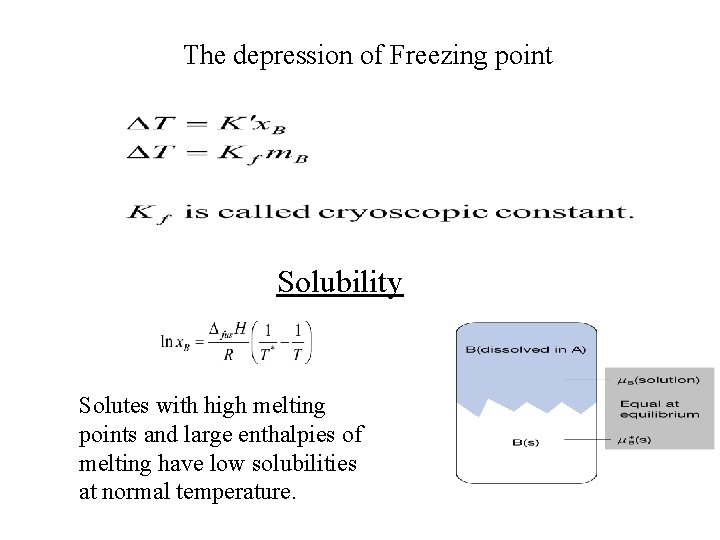
The depression of Freezing point Solubility Solutes with high melting points and large enthalpies of melting have low solubilities at normal temperature.

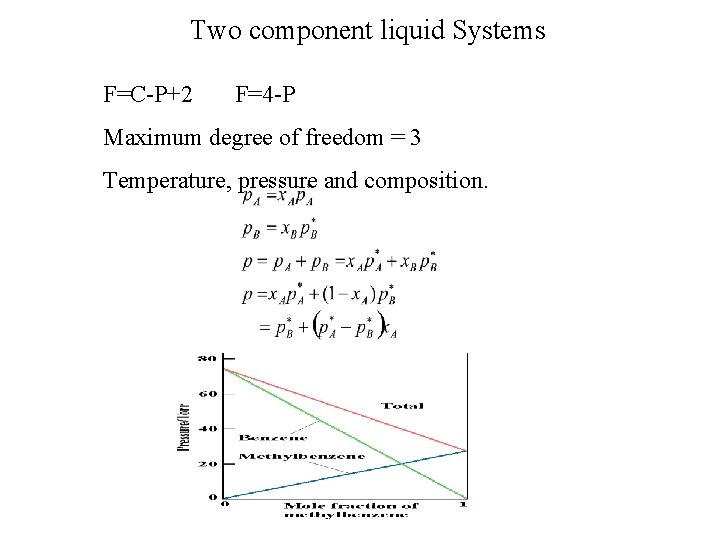
Two component liquid Systems F=C-P+2 F=4 -P Maximum degree of freedom = 3 Temperature, pressure and composition.



Q. One mole of benzene is mixed with two moles of toluene. At 60 C , the vapor pressure of benzene and toluene are 51. 3 and 18. 5 k. Pa, respectively. (a) As the pressure is reduced, at what pressure will boiling begin? (b) What will be the composition of the first bubble of vapor?

 Nr-13
Nr-13 The vapor pressure of pure water at 110 c is 1070 torr
The vapor pressure of pure water at 110 c is 1070 torr Pure solvent
Pure solvent Which of the following involves a colligative property
Which of the following involves a colligative property Exercise
Exercise Shortcut vapor pressure equation
Shortcut vapor pressure equation Vapour pressure lowering
Vapour pressure lowering Explain depression in freezing point
Explain depression in freezing point How to calculate vapor pressure of water
How to calculate vapor pressure of water London dispersion forces diagram
London dispersion forces diagram Chem 150
Chem 150 Vapor pressure and boiling worksheet answers
Vapor pressure and boiling worksheet answers Hemolysis and crenation
Hemolysis and crenation Bu vle
Bu vle Vapor pressure
Vapor pressure Negative deviation from raoult's law
Negative deviation from raoult's law Clausius clapeyron formula
Clausius clapeyron formula Chloride shift
Chloride shift Founder effect vs bottleneck effect
Founder effect vs bottleneck effect Substitution effect and income effect
Substitution effect and income effect Phản ứng thế ankan
Phản ứng thế ankan