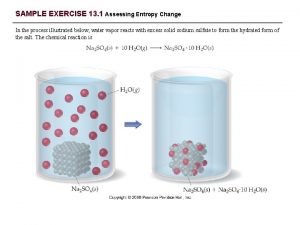
Liquids Vapor Pressure Vapor Pressure VP Pressure exerted








- Slides: 14

Liquids & Vapor Pressure

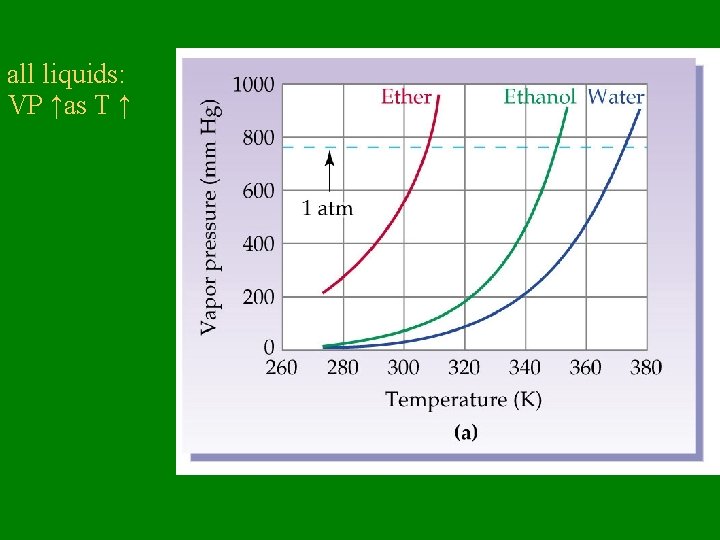
Vapor Pressure (VP) = Pressure exerted by vapor over its liquid How is vapor pressure affected by temperature? • higher the temperature, the higher the VP

Vapor • Gas phase of substance that is normally liquid at room temperature Evaporation occurs at all temperatures • the easier a substance evaporates, the weaker the attractive forces between liquid molecules

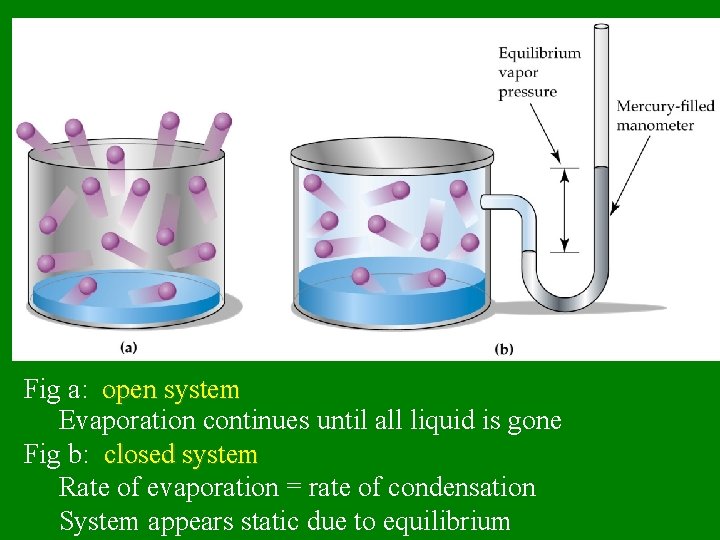
Fig a: open system Evaporation continues until all liquid is gone Fig b: closed system Rate of evaporation = rate of condensation System appears static due to equilibrium

all liquids: VP ↑as T ↑

Vapor Pressure • VP does NOT depend on how much liquid is present • VP depends ONLY on temperature

Phase Changes 1. Melting s → l 2. Freezing l → s 3. Vaporization (Boiling) l → g - occurs throughout liquid, temp stays constant Evaporation - occurs at surface; temperature drops 4. Condensation g → l 5. Deposition g → s 6. Sublimation s → g

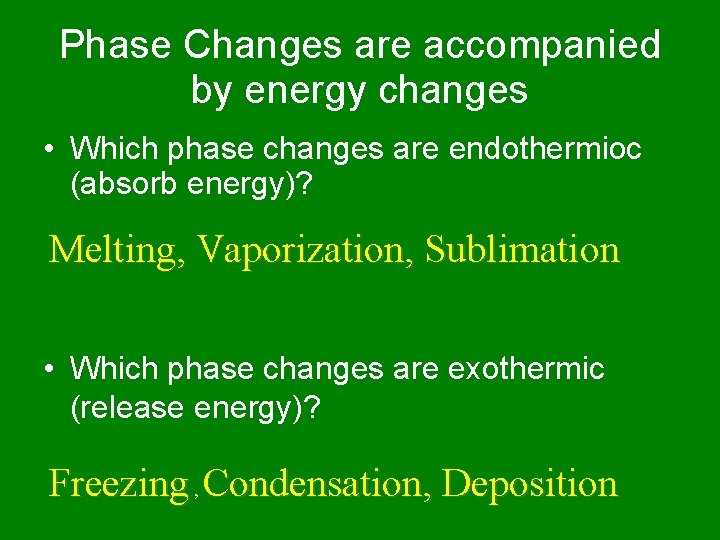
Phase Changes are accompanied by energy changes • Which phase changes are endothermioc (absorb energy)? Melting, Vaporization, Sublimation • Which phase changes are exothermic (release energy)? Freezing , Condensation, Deposition

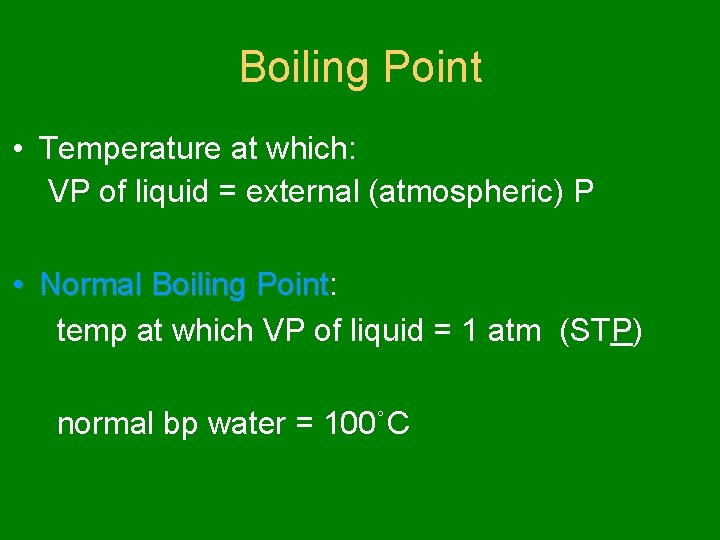
Boiling Point • Temperature at which: VP of liquid = external (atmospheric) P • Normal Boiling Point: Point temp at which VP of liquid = 1 atm (STP) normal bp water = 100˚C


Melting Point • = temp at which liquid and solid phases of substance coexist at equilibrium normal mp water = 0˚C

Freezing Point • Temp at which liquid is converted to crystalline solid • How does freezing point compare to melting point? They’re the same: for water = 0˚C

Boiling and Pressure • If ↑ external pressure (say are camping in Death Valley), the boiling point of water is ____ > than 100 o. C • If ↓ external pressure (say are eating Raman noodles at top of Mt. Whitney), the boiling o. C point of water is ____ than 100 <

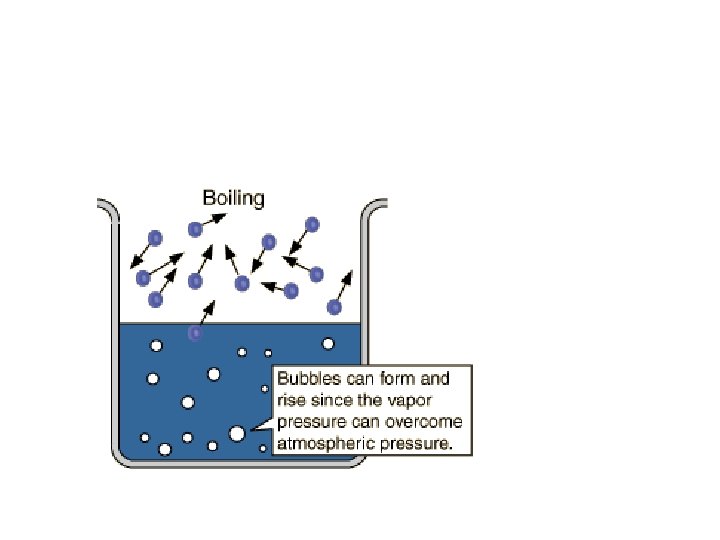
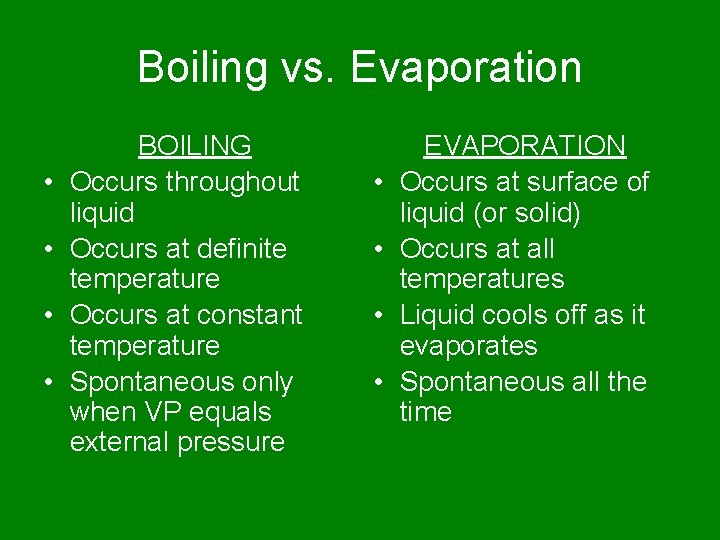
Boiling vs. Evaporation • • BOILING Occurs throughout liquid Occurs at definite temperature Occurs at constant temperature Spontaneous only when VP equals external pressure • • EVAPORATION Occurs at surface of liquid (or solid) Occurs at all temperatures Liquid cools off as it evaporates Spontaneous all the time
 Nr-13
Nr-13 In which direction is air pressure exerted?
In which direction is air pressure exerted? Calculate pressure exerted by a screw on the wooden plank
Calculate pressure exerted by a screw on the wooden plank Rocks changed by temperature pressure and hot liquids
Rocks changed by temperature pressure and hot liquids Why freezing point decreases on adding solute
Why freezing point decreases on adding solute Which of the following involves a colligative property
Which of the following involves a colligative property Glycerin vapor pressure
Glycerin vapor pressure Chem 150
Chem 150 Tb=kbm
Tb=kbm Explain depression in freezing point
Explain depression in freezing point Water vapour formula
Water vapour formula Phase diagram of carbon dioxide
Phase diagram of carbon dioxide Vapor pressure worksheet
Vapor pressure worksheet Raoult's law and dalton's law
Raoult's law and dalton's law Hemolysis and crenation
Hemolysis and crenation