Structural Analysis 3 Gordon Watson Chemistry Department Kelso

- Slides: 43

Structural Analysis 3 Gordon Watson Chemistry Department, Kelso High School Adv Higher Unit 3 Topic 4 KHS Chemistry Unit 3. 4 Structural Analysis 1

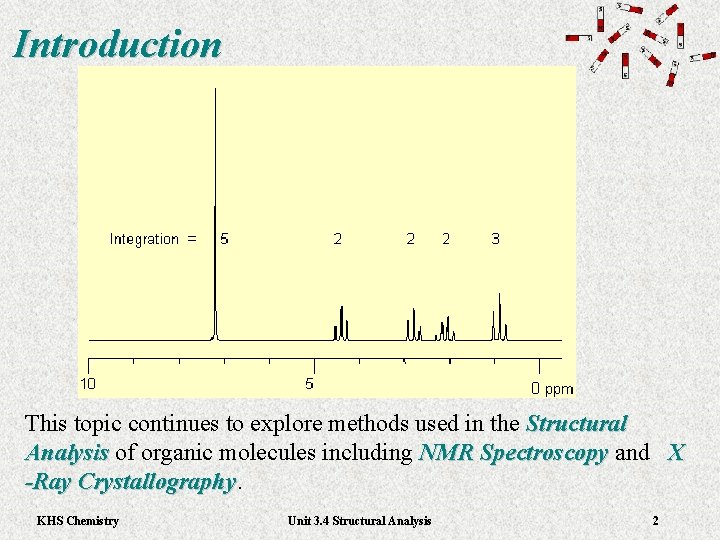
Introduction This topic continues to explore methods used in the Structural Analysis of organic molecules including NMR Spectroscopy and X Analysis NMR Spectroscopy -Ray Crystallography KHS Chemistry Unit 3. 4 Structural Analysis 2

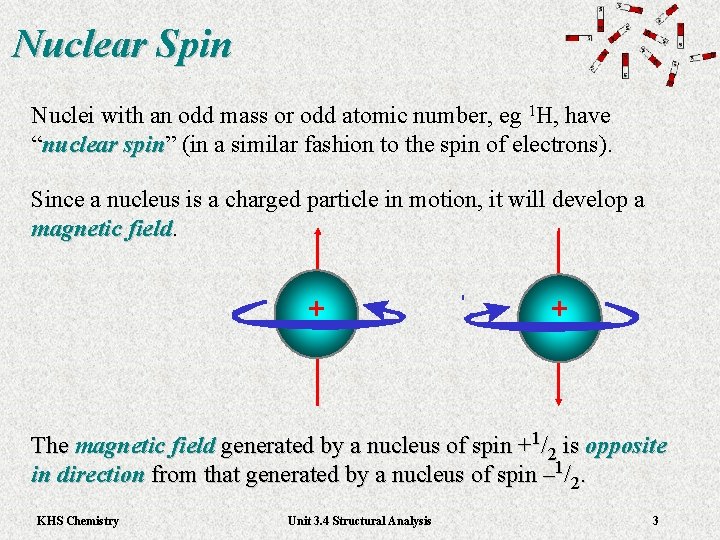
Nuclear Spin Nuclei with an odd mass or odd atomic number, eg 1 H, have “nuclear spin” (in a similar fashion to the spin of electrons). nuclear spin Since a nucleus is a charged particle in motion, it will develop a magnetic field + + The magnetic field generated by a nucleus of spin +1/2 is opposite in direction from that generated by a nucleus of spin – 1/2. KHS Chemistry Unit 3. 4 Structural Analysis 3

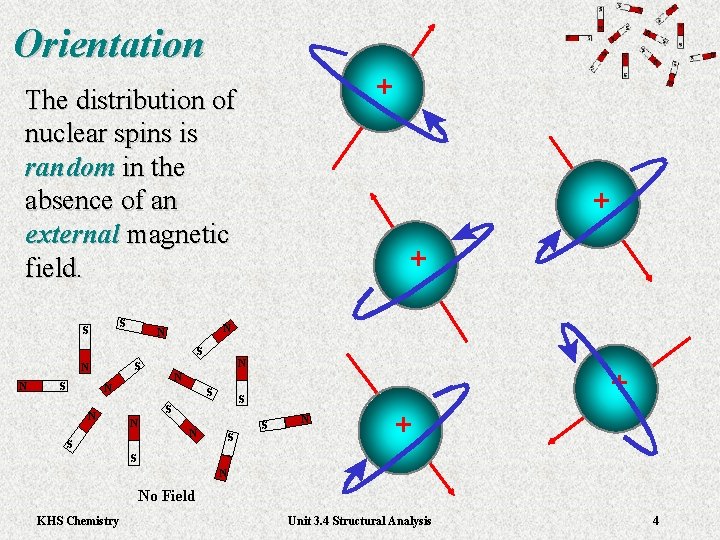
Orientation The distribution of nuclear spins is random in the absence of an external magnetic field. + + + KHS Chemistry Unit 3. 4 Structural Analysis 4

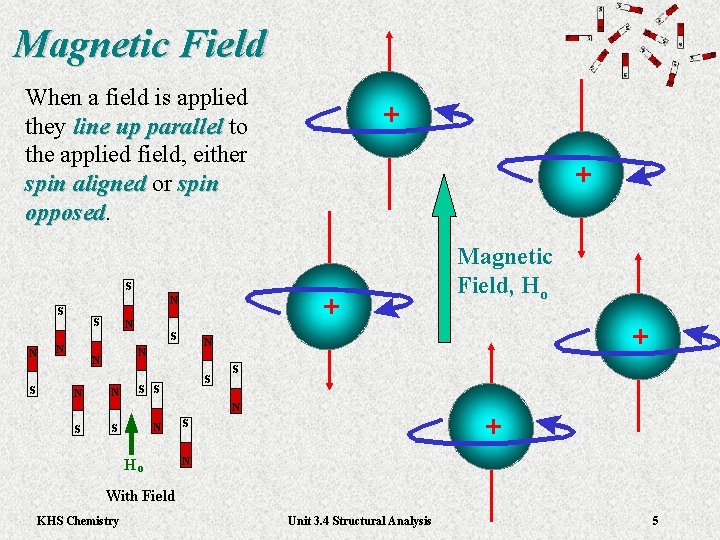
Magnetic Field When a field is applied they line up parallel to line up parallel the applied field, either spin aligned or spin aligned spin opposed + + Magnetic Field, Ho + + + KHS Chemistry Unit 3. 4 Structural Analysis 5

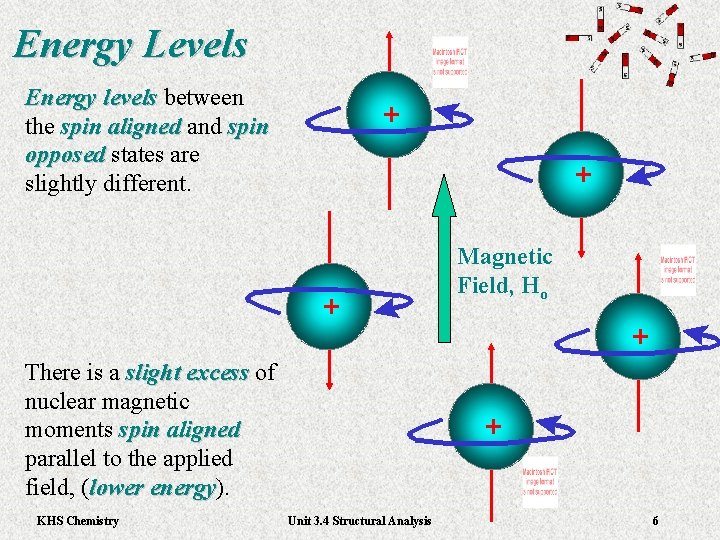
Energy Levels Energy levels between Energy levels the spin aligned and spin aligned opposed states are opposed slightly different. + + + There is a slight excess of slight excess nuclear magnetic moments spin aligned parallel to the applied field, (lower energy). lower energy KHS Chemistry Magnetic Field, Ho + + Unit 3. 4 Structural Analysis 6

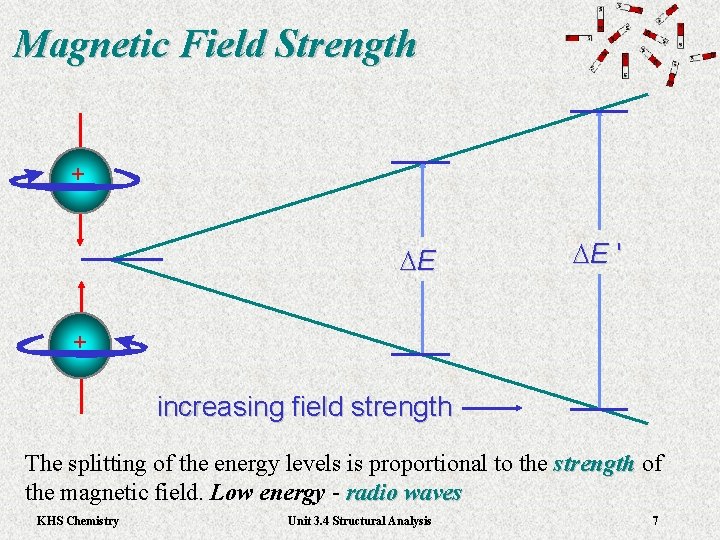
Magnetic Field Strength + DE DE ' + increasing field strength The splitting of the energy levels is proportional to the strength of strength the magnetic field. Low energy - radio waves KHS Chemistry Unit 3. 4 Structural Analysis 7

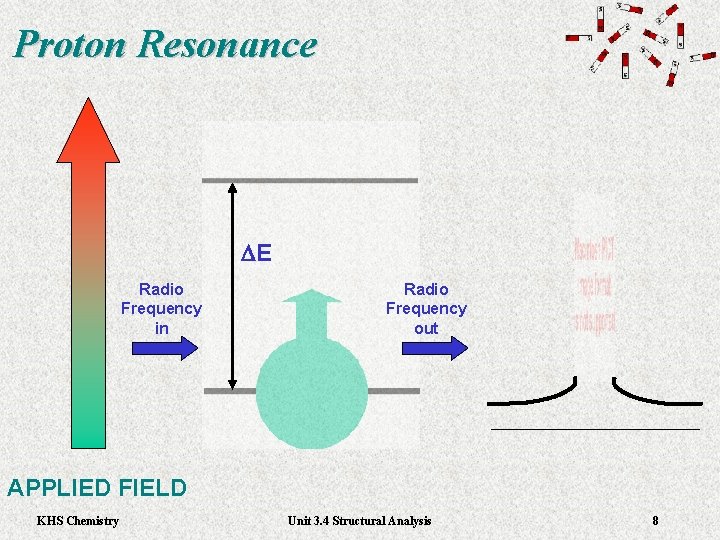
Proton Resonance DE Radio Frequency in Radio Frequency out APPLIED FIELD KHS Chemistry Unit 3. 4 Structural Analysis 8

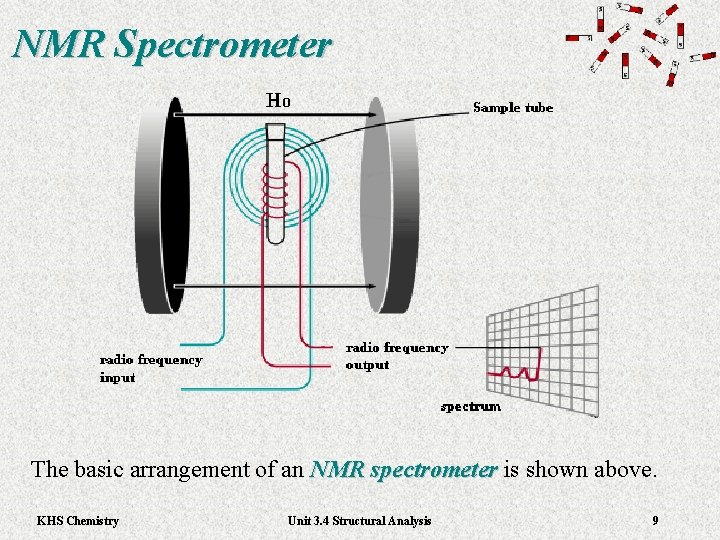
NMR Spectrometer The basic arrangement of an NMR spectrometer is shown above. NMR spectrometer KHS Chemistry Unit 3. 4 Structural Analysis 9

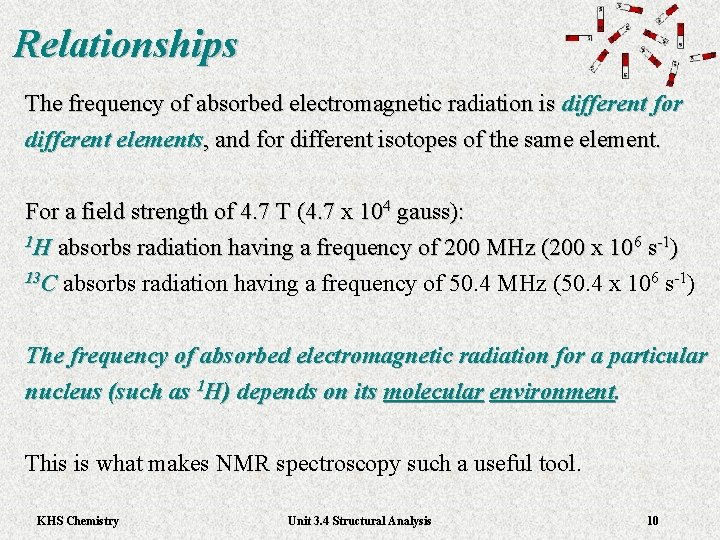
Relationships The frequency of absorbed electromagnetic radiation is different for different elements, and for different isotopes of the same element. For a field strength of 4. 7 T (4. 7 x 104 gauss): 1 H absorbs radiation having a frequency of 200 MHz (200 x 106 s-1) 13 C absorbs radiation having a frequency of 50. 4 MHz (50. 4 x 106 s-1) The frequency of absorbed electromagnetic radiation for a particular nucleus (such as 1 H) depends on its molecular environment. This is what makes NMR spectroscopy such a useful tool. KHS Chemistry Unit 3. 4 Structural Analysis 10

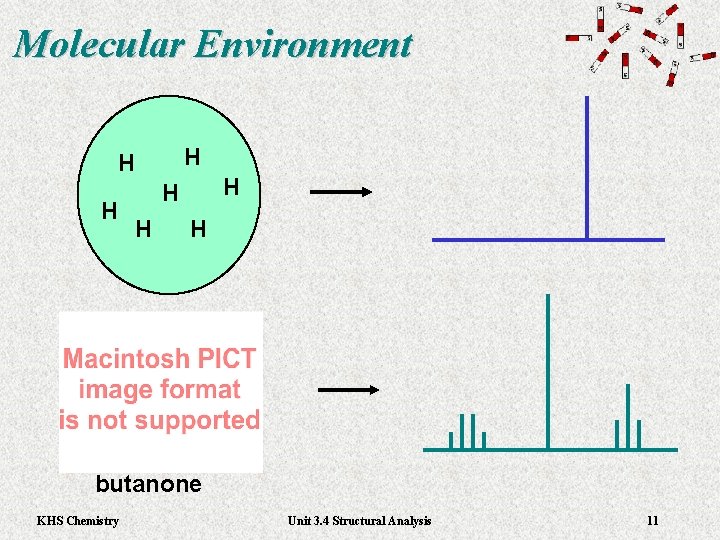
Molecular Environment H H H H butanone KHS Chemistry Unit 3. 4 Structural Analysis 11

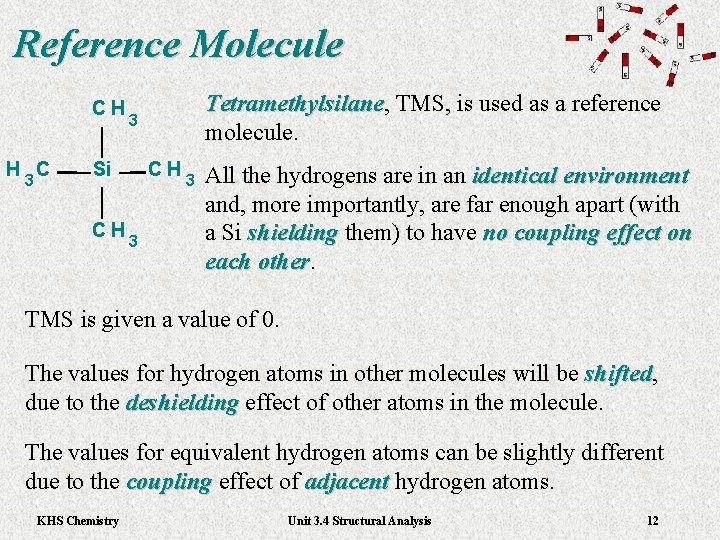
Reference Molecule CH H C 3 3 Si CH Tetramethylsilane, TMS, is used as a reference Tetramethylsilane molecule. CH 3 3 All the hydrogens are in an identical environment and, more importantly, are far enough apart (with a Si shielding them) to have no coupling effect on shielding each other TMS is given a value of 0. The values for hydrogen atoms in other molecules will be shifted, shifted due to the deshielding effect of other atoms in the molecule. deshielding The values for equivalent hydrogen atoms can be slightly different due to the coupling effect of adjacent hydrogen atoms. coupling adjacent KHS Chemistry Unit 3. 4 Structural Analysis 12

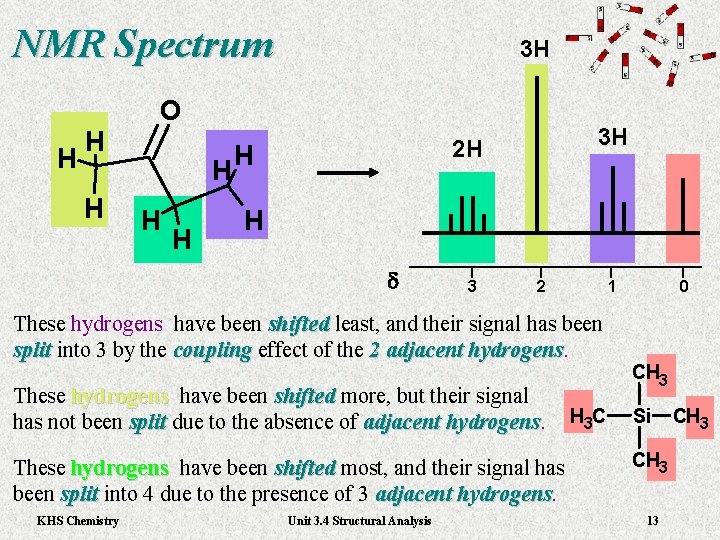
NMR Spectrum H H H 3 H O H H H 3 H 2 H H H d 3 2 1 These hydrogens have been shifted least, and their signal has been shifted split into 3 by the coupling effect of the 2 adjacent hydrogens. split coupling hydrogens These hydrogens have been shifted more, but their signal hydrogens shifted has not been split due to the absence of adjacent hydrogens. split hydrogens H 3 C These hydrogens have been shifted most, and their signal has hydrogens shifted been split into 4 due to the presence of 3 adjacent hydrogens. split hydrogens KHS Chemistry Unit 3. 4 Structural Analysis 0 CH 3 Si CH 3 13 CH 3

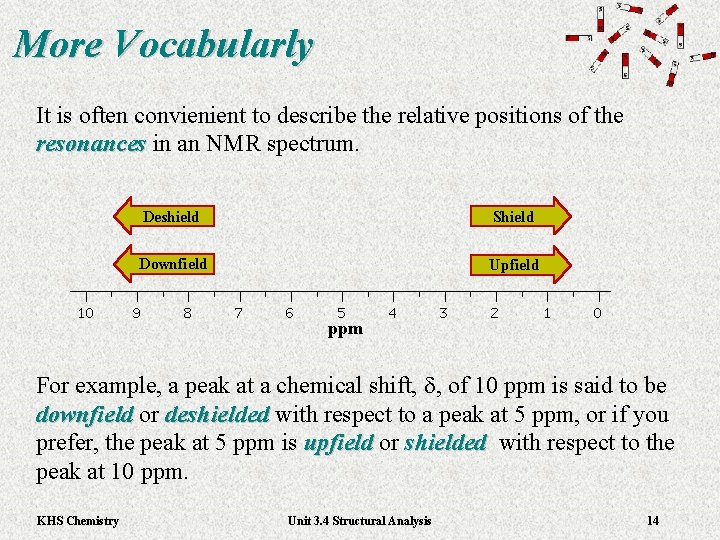
More Vocabularly It is often convienient to describe the relative positions of the resonances in an NMR spectrum. resonances For example, a peak at a chemical shift, d, of 10 ppm is said to be downfield or downfield deshielded with respect to a peak at 5 ppm, or if you deshielded prefer, the peak at 5 ppm is upfield or upfield shielded with respect to the shielded peak at 10 ppm. KHS Chemistry Unit 3. 4 Structural Analysis 14

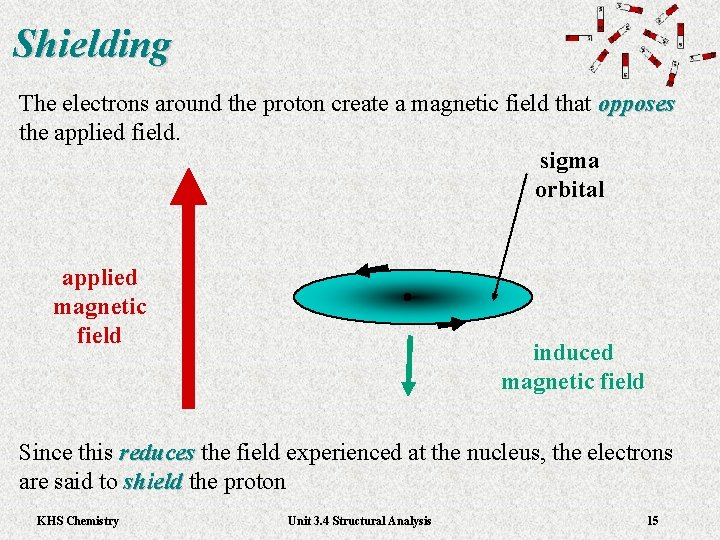
Shielding The electrons around the proton create a magnetic field that opposes the applied field. sigma orbital applied magnetic field induced magnetic field Since this reduces the field experienced at the nucleus, the electrons reduces are said to shield the proton shield KHS Chemistry Unit 3. 4 Structural Analysis 15

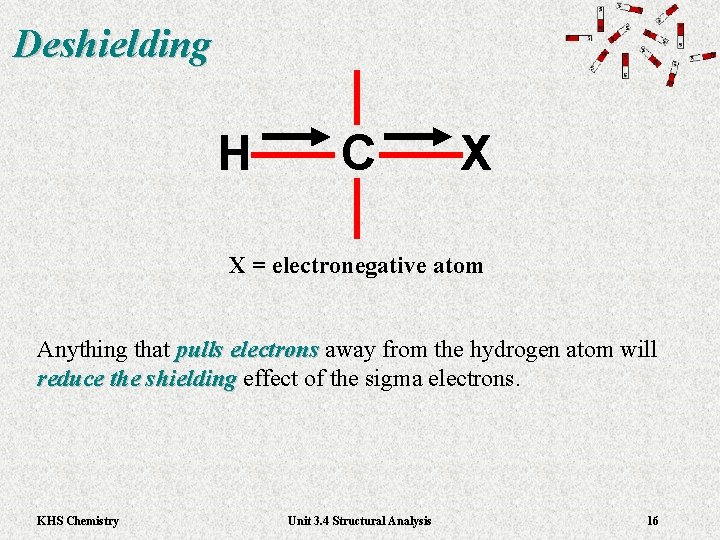
Deshielding H C X X = electronegative atom Anything that pulls electrons away from the hydrogen atom will pulls electrons reduce the shielding effect of the sigma electrons. reduce the shielding KHS Chemistry Unit 3. 4 Structural Analysis 16

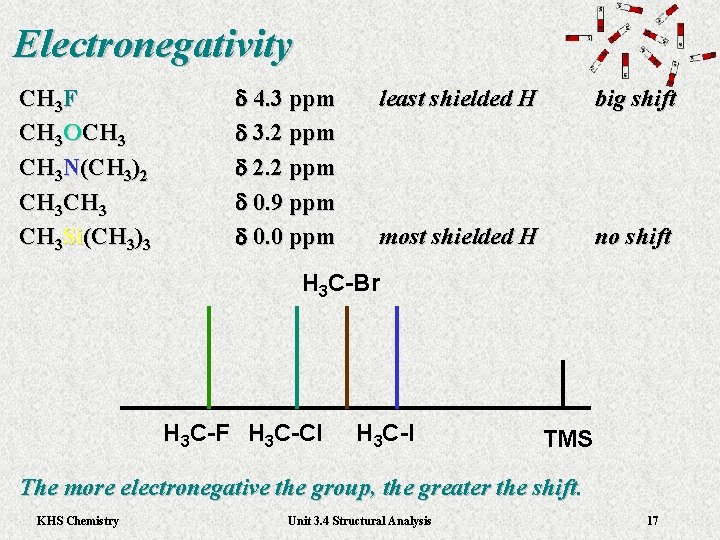
Electronegativity CH 3 F CH 3 OCH 3 N(CH 3)2 CH 3 Si(CH 3)3 d 4. 3 ppm d 3. 2 ppm d 2. 2 ppm d 0. 9 ppm d 0. 0 ppm least shielded H big shift most shielded H no shift H 3 C-Br H 3 C-F H 3 C-Cl H 3 C-I TMS The more electronegative the group, the greater the shift. KHS Chemistry Unit 3. 4 Structural Analysis 17

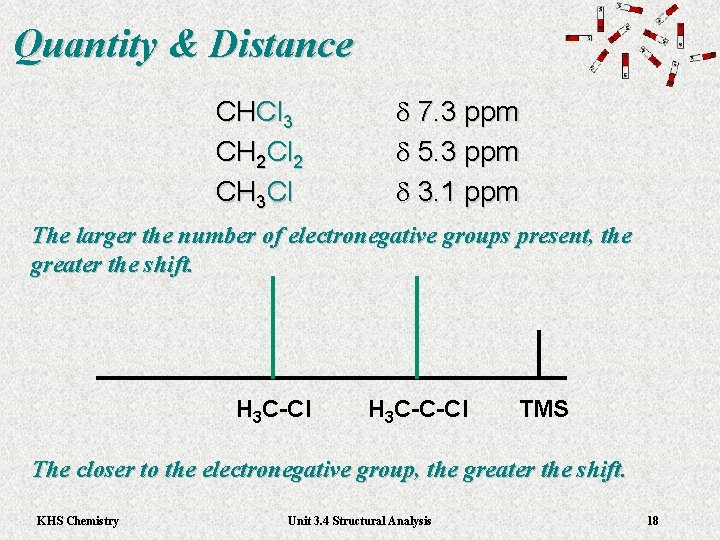
Quantity & Distance CHCl 3 CH 2 Cl 2 CH 3 Cl d 7. 3 ppm d 5. 3 ppm d 3. 1 ppm The larger the number of electronegative groups present, the greater the shift. H 3 C-Cl H 3 C-C-Cl TMS The closer to the electronegative group, the greater the shift. KHS Chemistry Unit 3. 4 Structural Analysis 18

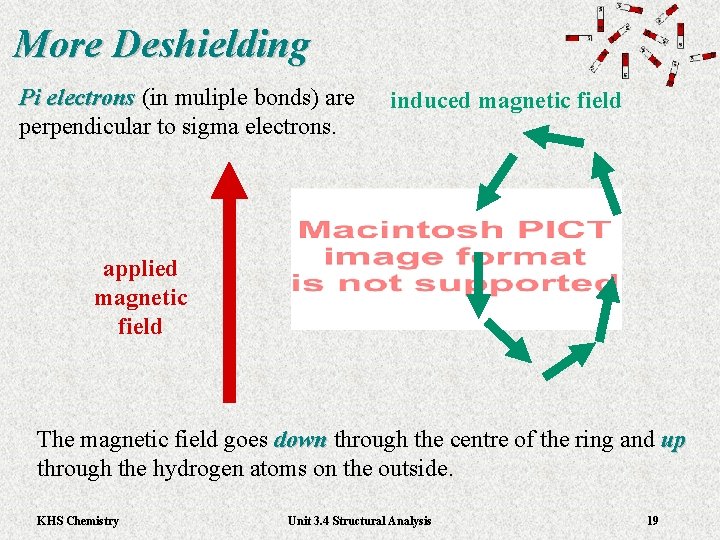
More Deshielding Pi electrons (in muliple bonds) are Pi electrons perpendicular to sigma electrons. induced magnetic field applied magnetic field The magnetic field goes down through the centre of the ring and up down through the hydrogen atoms on the outside. KHS Chemistry Unit 3. 4 Structural Analysis 19

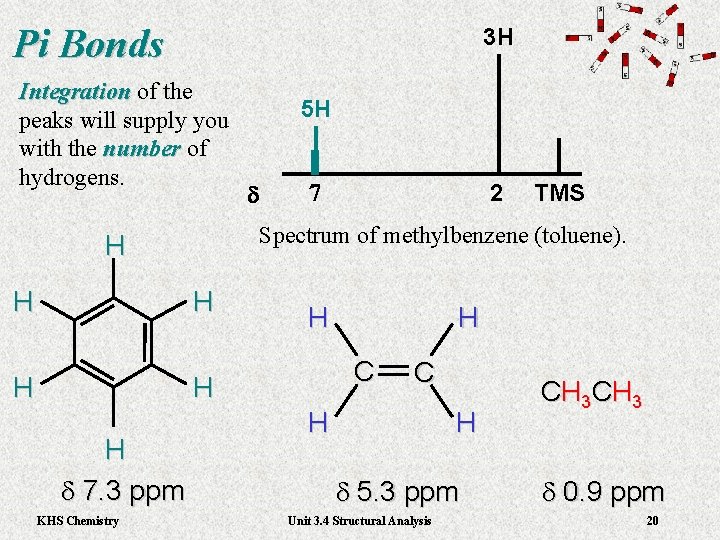
Pi Bonds 3 H Integration of the Integration peaks will supply you with the number of number hydrogens. H H d 7 H KHS Chemistry TMS H C H H d 7. 3 ppm 2 Spectrum of methylbenzene (toluene). H H 5 H C H H d 5. 3 ppm Unit 3. 4 Structural Analysis C H 3 d 0. 9 ppm 20

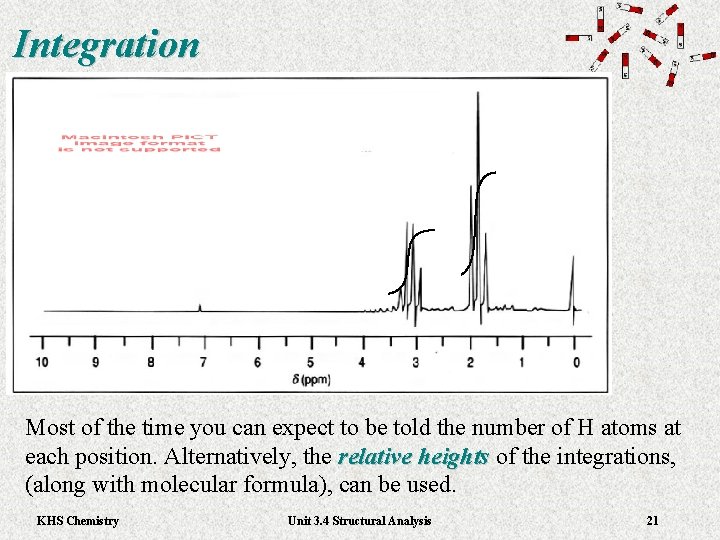
Integration Most of the time you can expect to be told the number of H atoms at each position. Alternatively, the relative heights of the integrations, relative heights (along with molecular formula), can be used. KHS Chemistry Unit 3. 4 Structural Analysis 21

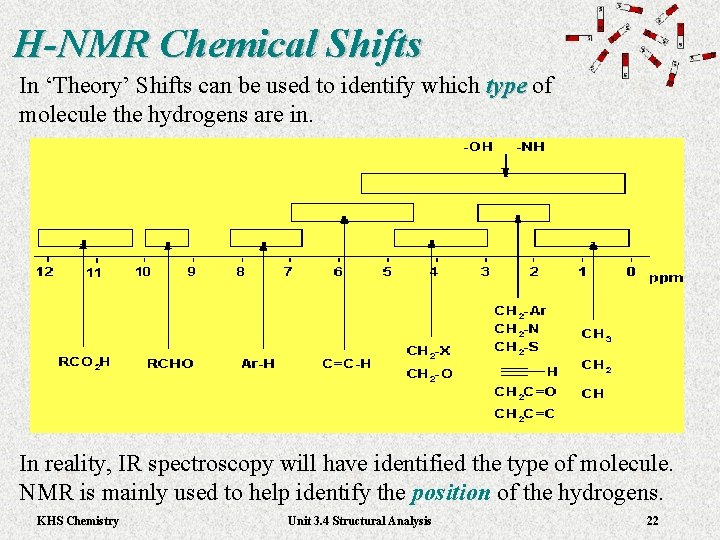
H-NMR Chemical Shifts In ‘Theory’ Shifts can be used to identify which type of type molecule the hydrogens are in. In reality, IR spectroscopy will have identified the type of molecule. NMR is mainly used to help identify the position of the hydrogens. KHS Chemistry Unit 3. 4 Structural Analysis 22

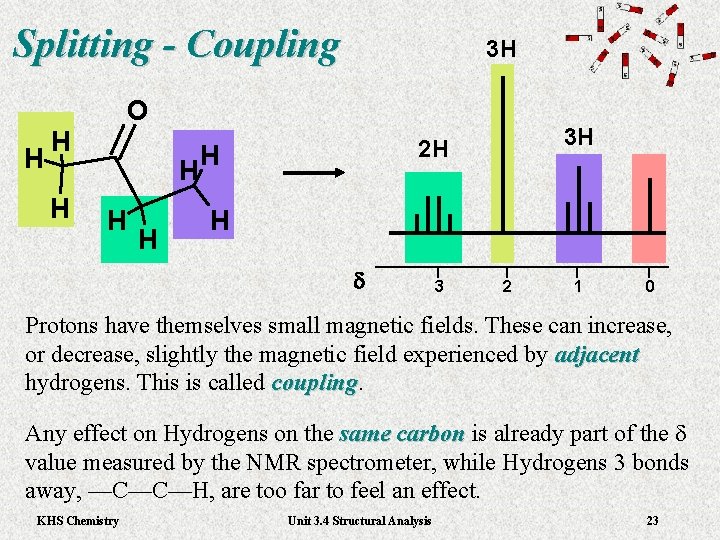
Splitting - Coupling H O H H 3 H 2 H H H d 3 2 1 0 Protons have themselves small magnetic fields. These can increase, or decrease, slightly the magnetic field experienced by adjacent hydrogens. This is called coupling Any effect on Hydrogens on the same carbon is already part of the d same carbon value measured by the NMR spectrometer, while Hydrogens 3 bonds away, —C—C—H, are too far to feel an effect. KHS Chemistry Unit 3. 4 Structural Analysis 23

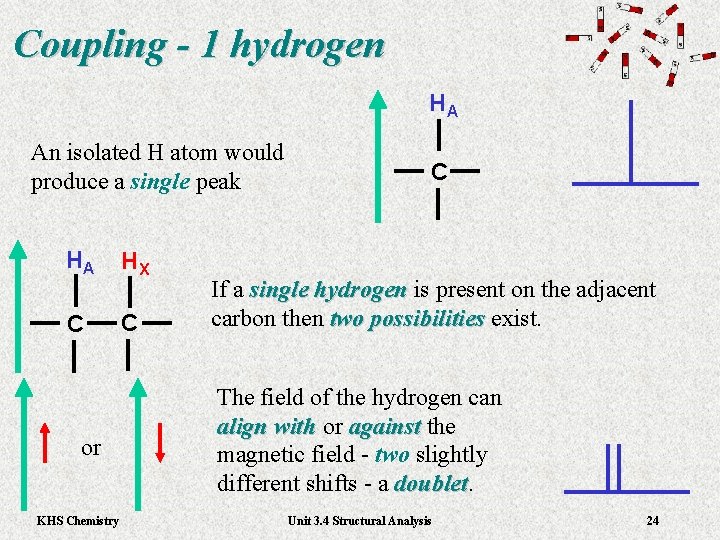
Coupling - 1 hydrogen HA An isolated H atom would produce a single peak HA HX C C or KHS Chemistry C If a single hydrogen is present on the adjacent single hydrogen carbon then two possibilities exist. two possibilities The field of the hydrogen can align with or align with against the against magnetic field - two slightly different shifts - a doublet Unit 3. 4 Structural Analysis 24

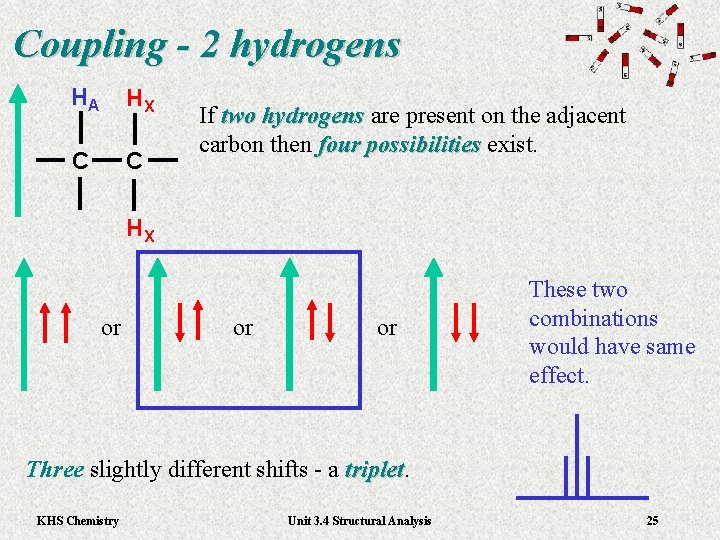
Coupling - 2 hydrogens HA HX C C If two hydrogens are present on the adjacent hydrogens carbon then four possibilities exist. four possibilities HX or or or These two combinations would have same effect. Three slightly different shifts - a triplet KHS Chemistry Unit 3. 4 Structural Analysis 25

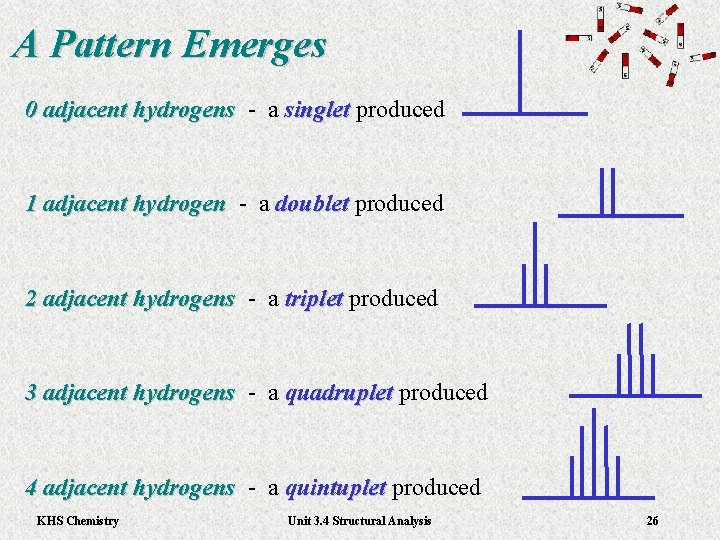
A Pattern Emerges 0 adjacent hydrogens - a hydrogens singlet produced singlet 1 adjacent hydrogen - a 1 adjacent hydrogen doublet produced doublet 2 adjacent hydrogens - a hydrogens triplet produced triplet 3 adjacent hydrogens - a hydrogens quadruplet produced quadruplet 4 adjacent hydrogens - a hydrogens quintuplet produced quintuplet KHS Chemistry Unit 3. 4 Structural Analysis 26

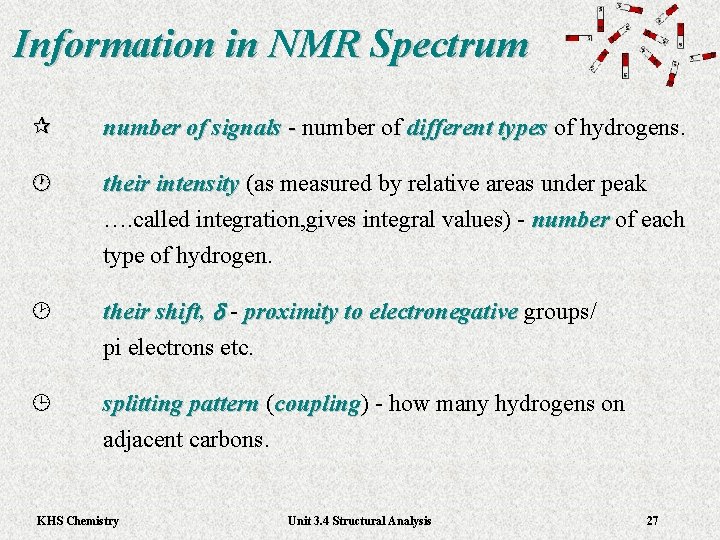
Information in NMR Spectrum ¶ number of signals - number of different types of hydrogens. - · their intensity (as measured by relative areas under peak their intensity …. called integration, gives integral values) - number of each number type of hydrogen. ¸ their shift, d - proximity to electronegative groups/ proximity to electronegative pi electrons etc. ¹ splitting pattern ( splitting pattern coupling) - how many hydrogens on coupling adjacent carbons. KHS Chemistry Unit 3. 4 Structural Analysis 27

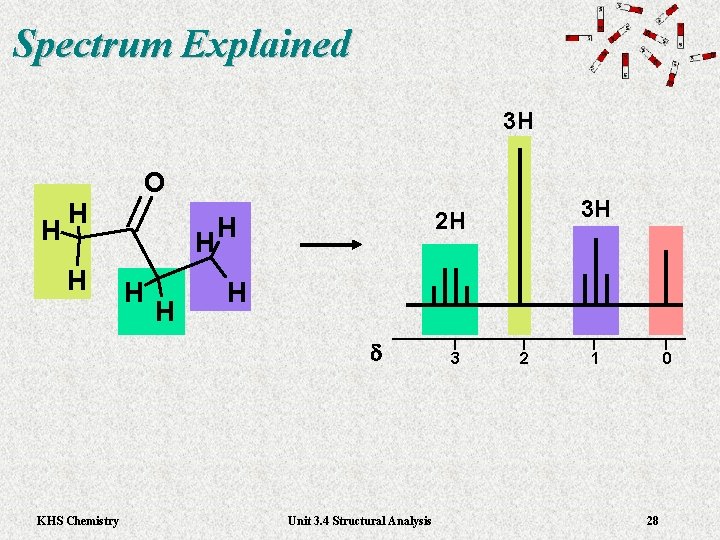
Spectrum Explained 3 H H O H H H d KHS Chemistry 3 H 2 H Unit 3. 4 Structural Analysis 3 2 1 0 28

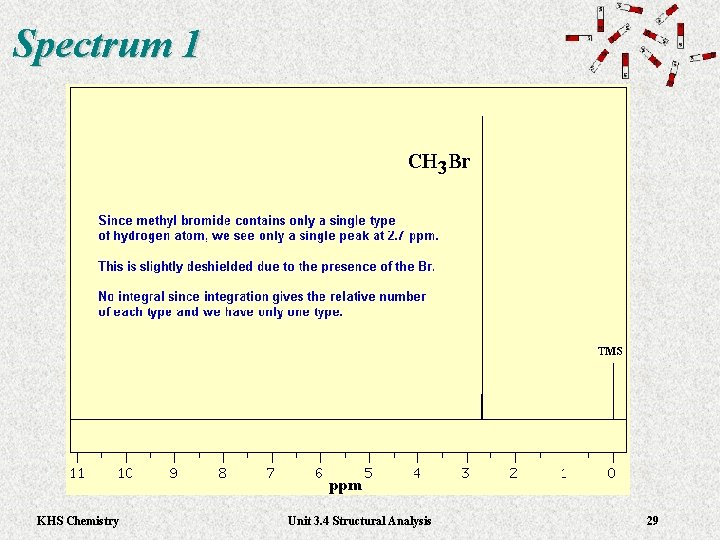
Spectrum 1 KHS Chemistry Unit 3. 4 Structural Analysis 29

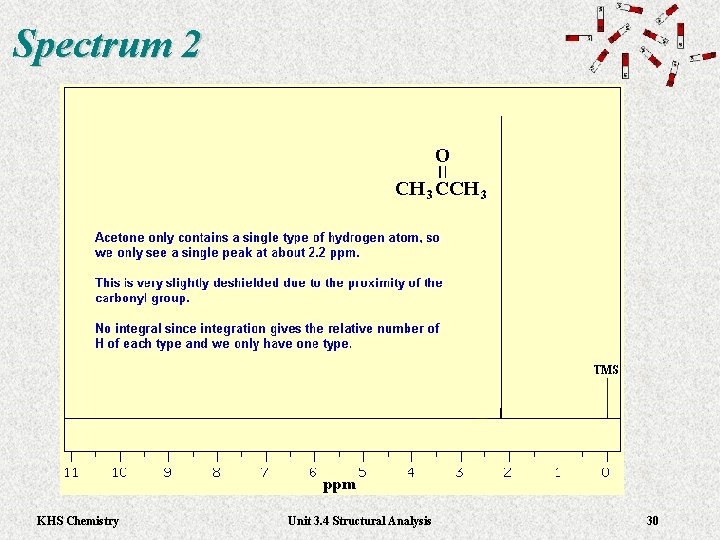
Spectrum 2 KHS Chemistry Unit 3. 4 Structural Analysis 30

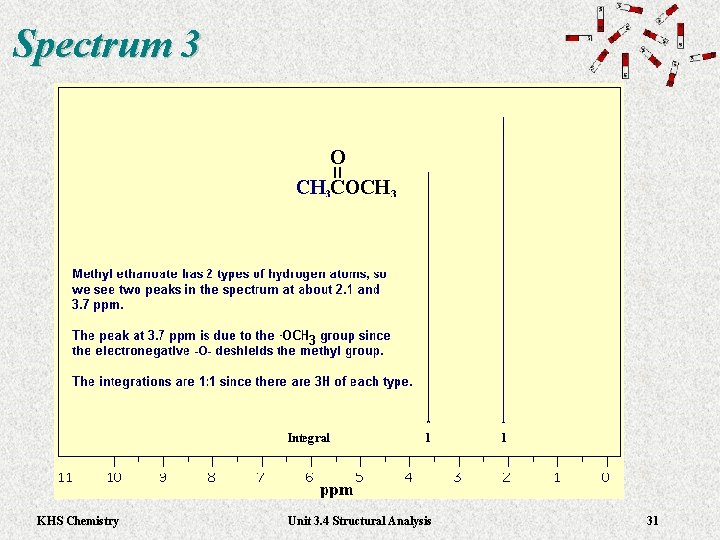
Spectrum 3 KHS Chemistry Unit 3. 4 Structural Analysis 31

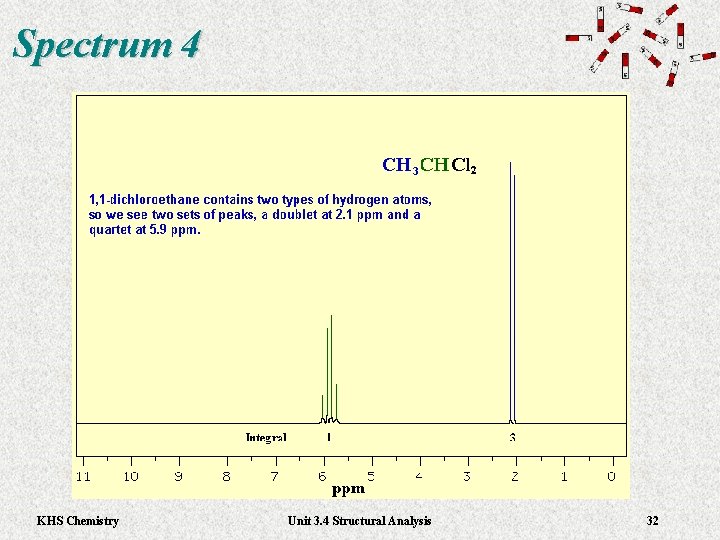
Spectrum 4 KHS Chemistry Unit 3. 4 Structural Analysis 32

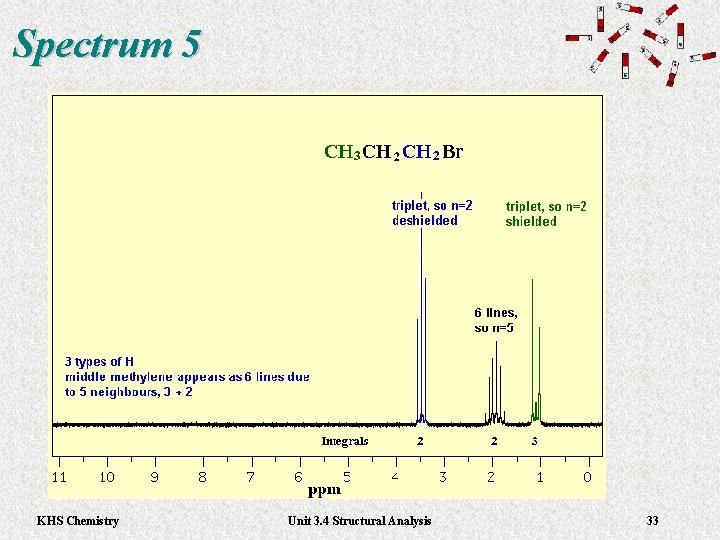
Spectrum 5 KHS Chemistry Unit 3. 4 Structural Analysis 33

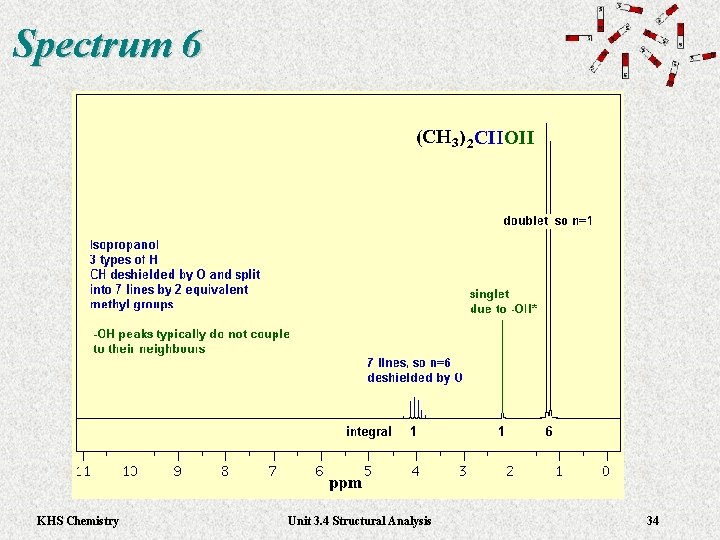
Spectrum 6 KHS Chemistry Unit 3. 4 Structural Analysis 34

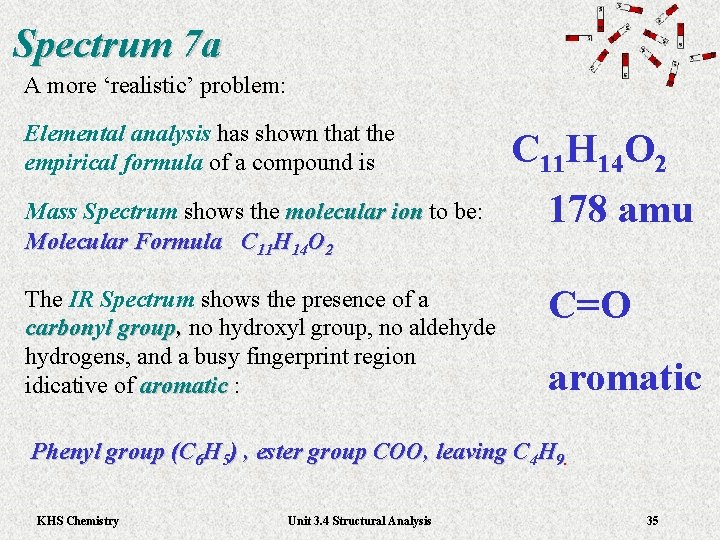
Spectrum 7 a A more ‘realistic’ problem: Elemental analysis has shown that the empirical formula of a compound is 11 14 C H O 2 Mass Spectrum shows the molecular ion to be: molecular ion Molecular Formula C 11 H 14 O 2 178 amu The IR Spectrum shows the presence of a carbonyl group, no hydroxyl group, no aldehyde , hydrogens, and a busy fingerprint region idicative of aromatic : aromatic C=O aromatic Phenyl group (C 6 H 5) , ester group COO, leaving C 4 H 9. KHS Chemistry Unit 3. 4 Structural Analysis 35

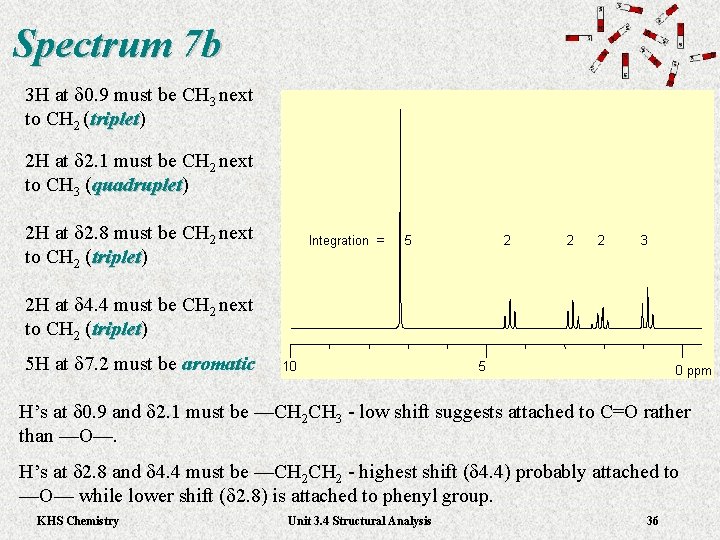
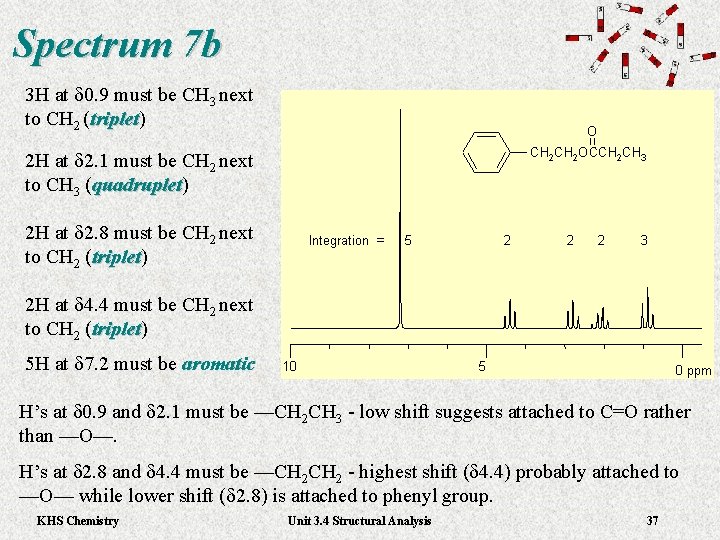
Spectrum 7 b 3 H at d 0. 9 must be CH 3 next to CH 2 (triplet) triplet 2 H at d 2. 1 must be CH 2 next to CH 3 (quadruplet) quadruplet 2 H at d 2. 8 must be CH 2 next to CH 2 (triplet) triplet 2 H at d 4. 4 must be CH 2 next to CH 2 (triplet) triplet 5 H at d 7. 2 must be aromatic H’s at d 0. 9 and d 2. 1 must be —CH 2 CH 3 - low shift suggests attached to C=O rather than —O—. H’s at d 2. 8 and d 4. 4 must be —CH 2 - highest shift (d 4. 4) probably attached to —O— while lower shift (d 2. 8) is attached to phenyl group. KHS Chemistry Unit 3. 4 Structural Analysis 36

Spectrum 7 b 3 H at d 0. 9 must be CH 3 next to CH 2 (triplet) triplet 2 H at d 2. 1 must be CH 2 next to CH 3 (quadruplet) quadruplet 2 H at d 2. 8 must be CH 2 next to CH 2 (triplet) triplet 2 H at d 4. 4 must be CH 2 next to CH 2 (triplet) triplet 5 H at d 7. 2 must be aromatic H’s at d 0. 9 and d 2. 1 must be —CH 2 CH 3 - low shift suggests attached to C=O rather than —O—. H’s at d 2. 8 and d 4. 4 must be —CH 2 - highest shift (d 4. 4) probably attached to —O— while lower shift (d 2. 8) is attached to phenyl group. KHS Chemistry Unit 3. 4 Structural Analysis 37

X-Rays KHS Chemistry Unit 3. 4 Structural Analysis 38

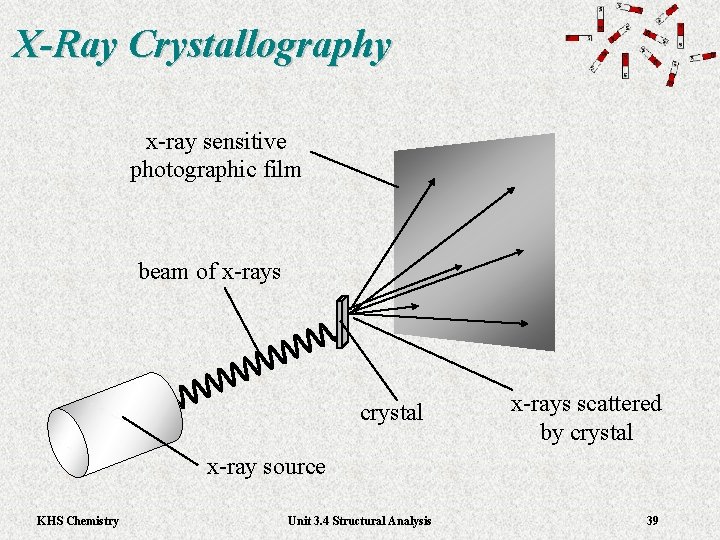
X-Ray Crystallography x-ray sensitive photographic film beam of x-rays crystal x-rays scattered by crystal x-ray source KHS Chemistry Unit 3. 4 Structural Analysis 39

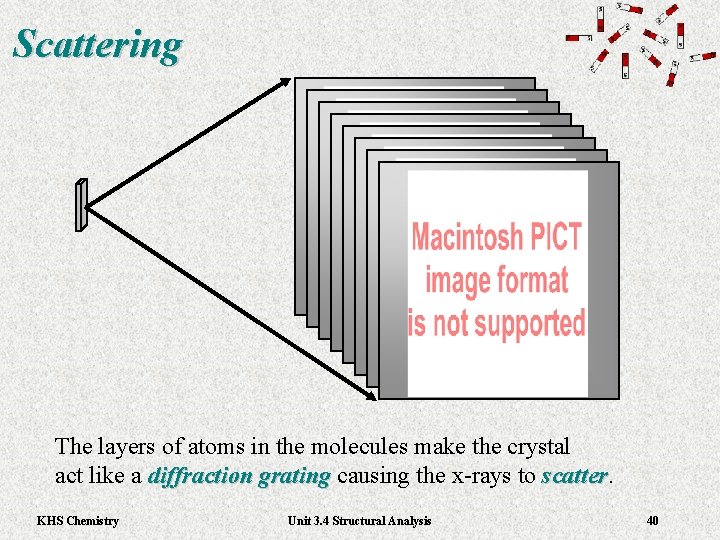
Scattering The layers of atoms in the molecules make the crystal act like a diffraction grating causing the x-rays to scatter. diffraction grating scatter KHS Chemistry Unit 3. 4 Structural Analysis 40

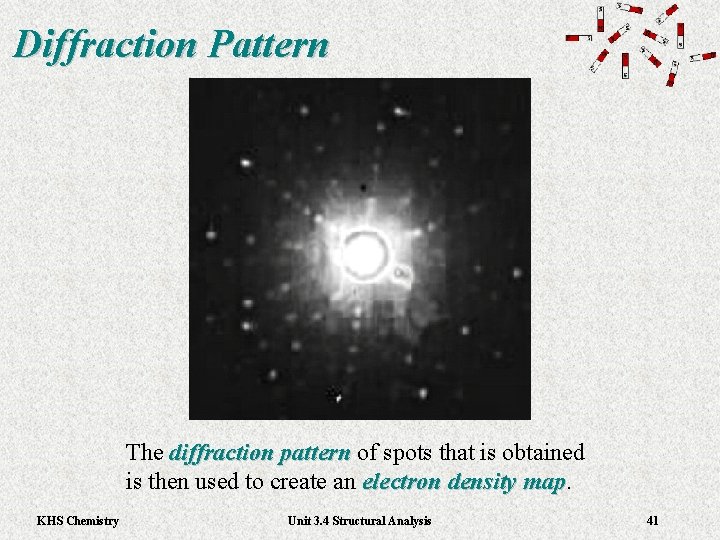
Diffraction Pattern The diffraction pattern of spots that is obtained diffraction pattern is then used to create an electron density map KHS Chemistry Unit 3. 4 Structural Analysis 41

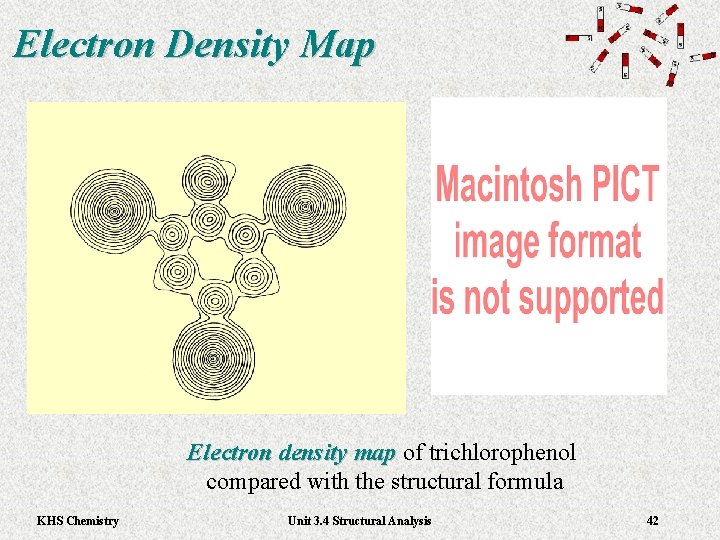
Electron Density Map Electron density map of trichlorophenol Electron density map compared with the structural formula KHS Chemistry Unit 3. 4 Structural Analysis 42

Structural Analysis 3 End of Topic 4 KHS Chemistry Unit 3. 4 Structural Analysis 43