Structural Analysis 2 Gordon Watson Chemistry Department Kelso


- Slides: 28

Structural Analysis 2 Gordon Watson Chemistry Department, Kelso High School Adv Higher Unit 3 Topic 4 KHS Chemistry Unit 3. 4 Structural Analysis 1

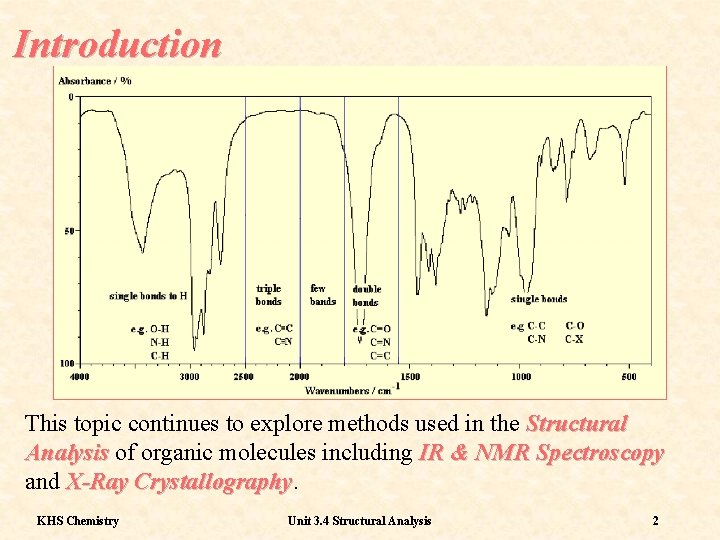
Introduction This topic continues to explore methods used in the Structural Analysis of organic molecules including IR & NMR Spectroscopy and X-Ray Crystallography KHS Chemistry Unit 3. 4 Structural Analysis 2

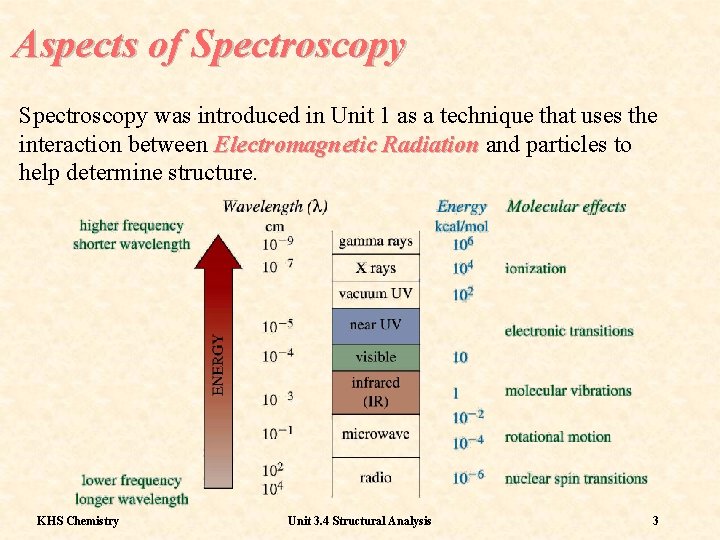
Aspects of Spectroscopy was introduced in Unit 1 as a technique that uses the interaction between Electromagnetic Radiation and particles to help determine structure. KHS Chemistry Unit 3. 4 Structural Analysis 3

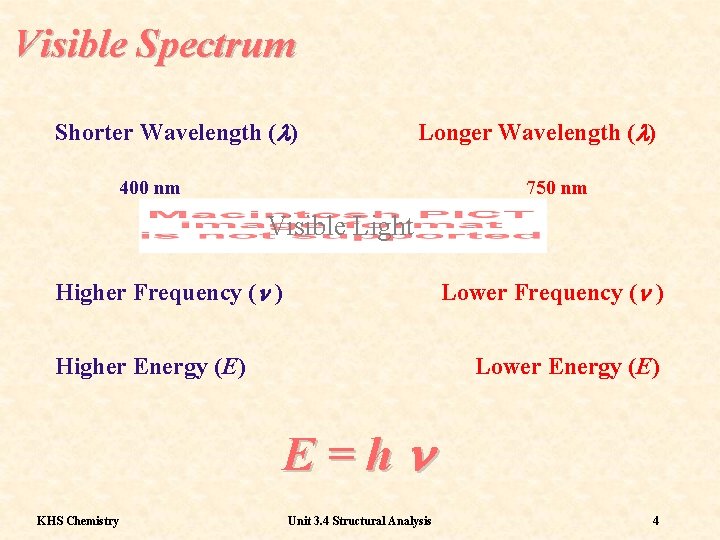
Visible Spectrum Shorter Wavelength (l) Longer Wavelength (l) 400 nm 750 nm Visible Light Higher Frequency (n ) Lower Frequency (n ) Higher Energy (E) Lower Energy (E) E=hn KHS Chemistry Unit 3. 4 Structural Analysis 4

Infra-Red Radiation Just below red in the visible region. Wavelengths usually 2500 -25000 nm. Visible Light. Infrared Ultraviolet IR Spectroscopy uses units called wavenumbers ( ), nu the reciprocal of the wavelength, (1/l), in centimeters (cm-1). Wavenumbers usually 4000 -400 cm-1 Wavenumbers are proportional to frequency and energy KHS Chemistry Unit 3. 4 Structural Analysis 5

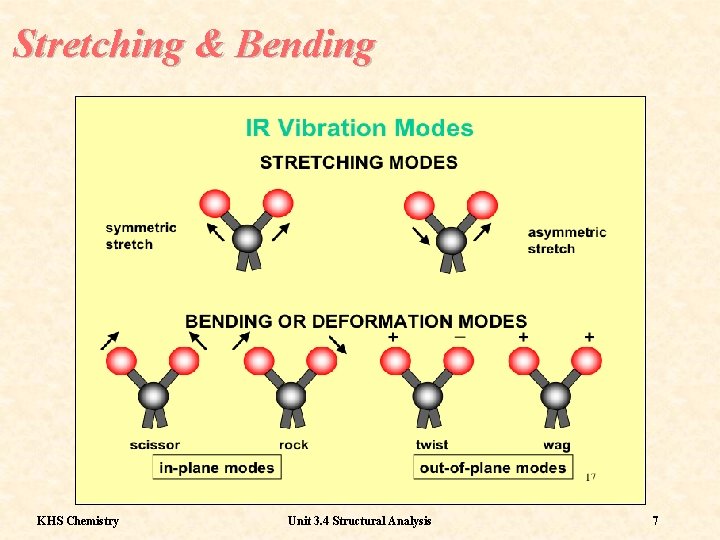
Molecular Vibrations Molecules have a variety of possible vibration states Some of these states are due to Stretching: Stretching which changes the distance between atoms in the molecule. Some of these states are due to Bending: Bending which changes the angle between atoms in the molecule. KHS Chemistry Unit 3. 4 Structural Analysis 6

Stretching & Bending KHS Chemistry Unit 3. 4 Structural Analysis 7

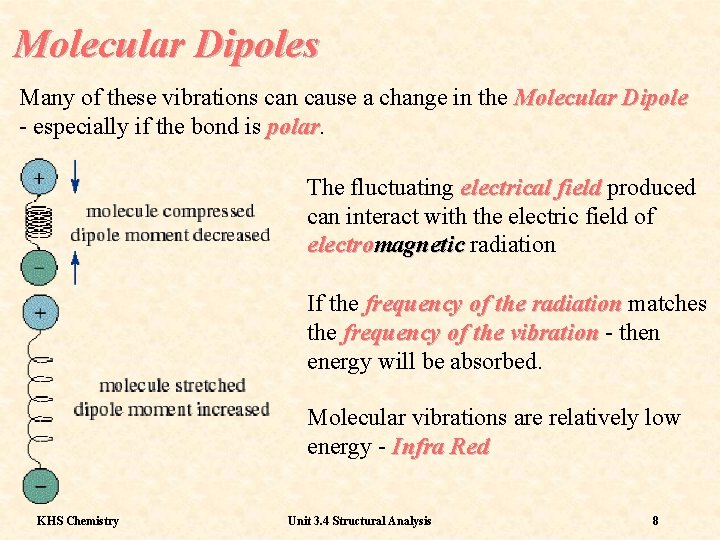
Molecular Dipoles Many of these vibrations can cause a change in the Molecular Dipole - especially if the bond is polar The fluctuating electrical field produced can interact with the electric field of electromagnetic radiation If the frequency of the radiation matches the frequency of the vibration - then energy will be absorbed. Molecular vibrations are relatively low energy - Infra Red KHS Chemistry Unit 3. 4 Structural Analysis 8

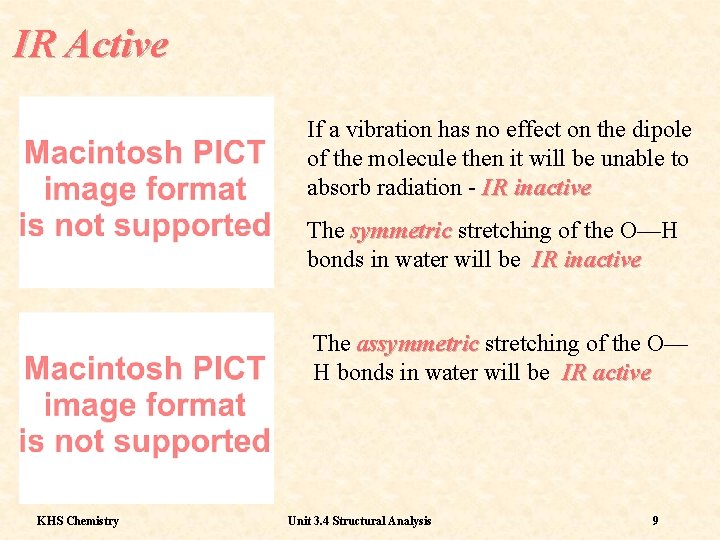
IR Active If a vibration has no effect on the dipole of the molecule then it will be unable to absorb radiation - IR inactive The symmetric stretching of the O—H bonds in water will be IR inactive The assymmetric stretching of the O— H bonds in water will be IR active KHS Chemistry Unit 3. 4 Structural Analysis 9

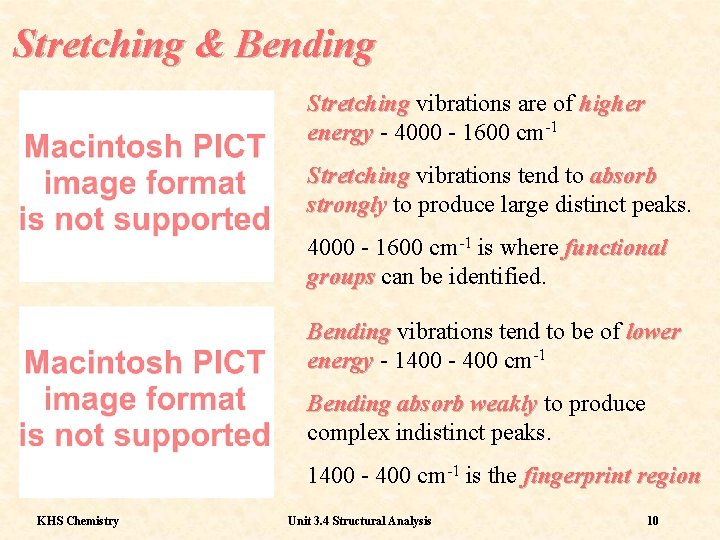
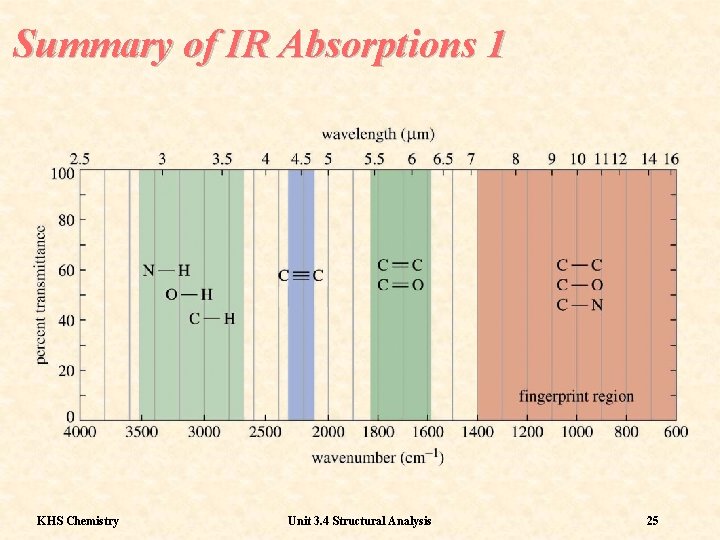
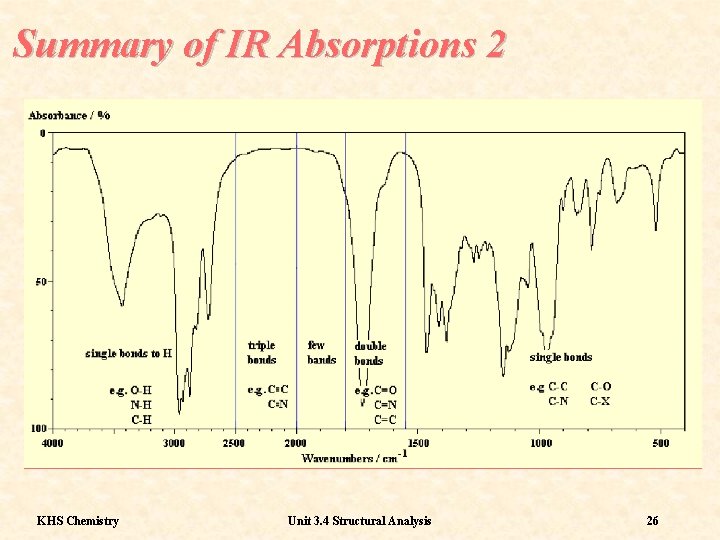
Stretching & Bending Stretching vibrations are of higher energy - 4000 - 1600 cm-1 Stretching vibrations tend to absorb strongly to produce large distinct peaks. 4000 - 1600 cm-1 is where functional groups can be identified. Bending vibrations tend to be of lower energy - 1400 - 400 cm-1 Bending absorb weakly to produce complex indistinct peaks. 1400 - 400 cm-1 is the fingerprint region KHS Chemistry Unit 3. 4 Structural Analysis 10

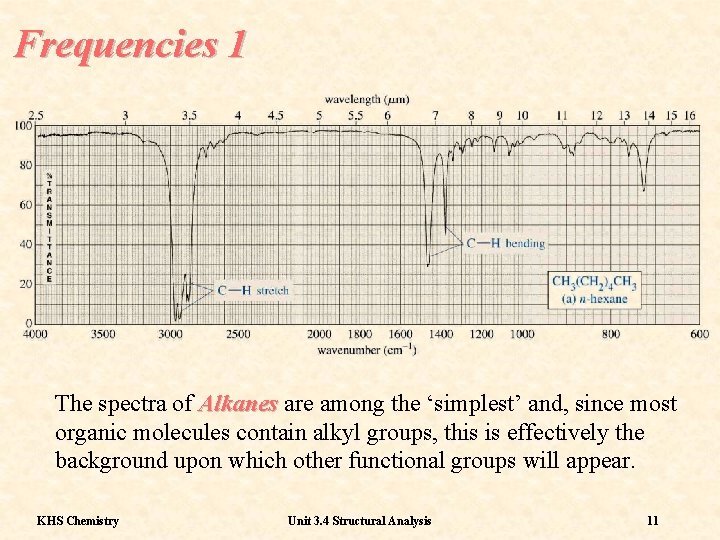
Frequencies 1 The spectra of Alkanes are among the ‘simplest’ and, since most organic molecules contain alkyl groups, this is effectively the background upon which other functional groups will appear. KHS Chemistry Unit 3. 4 Structural Analysis 11

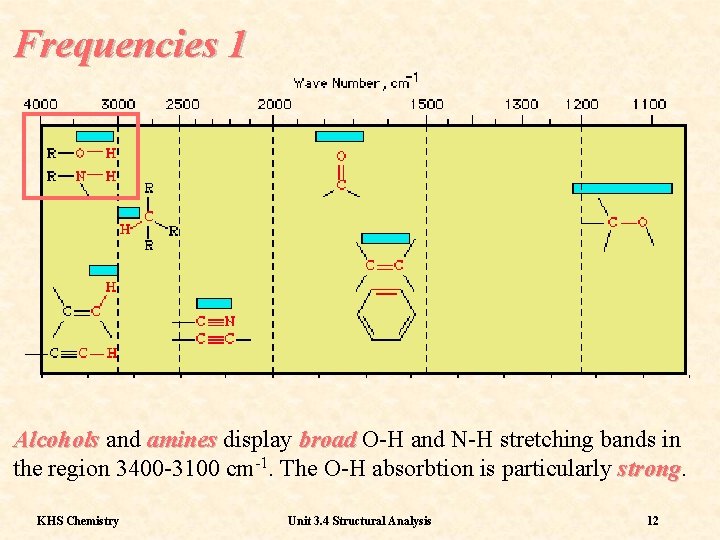
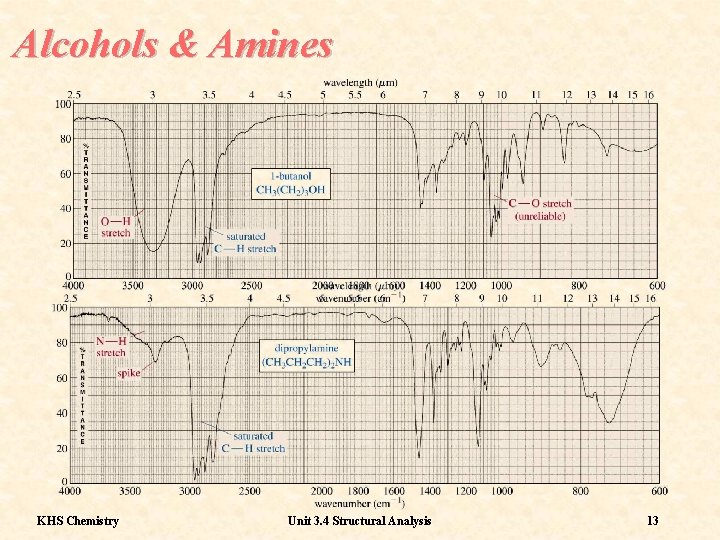
Frequencies 1 Alcohols and amines display broad O-H and N-H stretching bands in the region 3400 -3100 cm-1. The O-H absorbtion is particularly strong KHS Chemistry Unit 3. 4 Structural Analysis 12

Alcohols & Amines KHS Chemistry Unit 3. 4 Structural Analysis 13

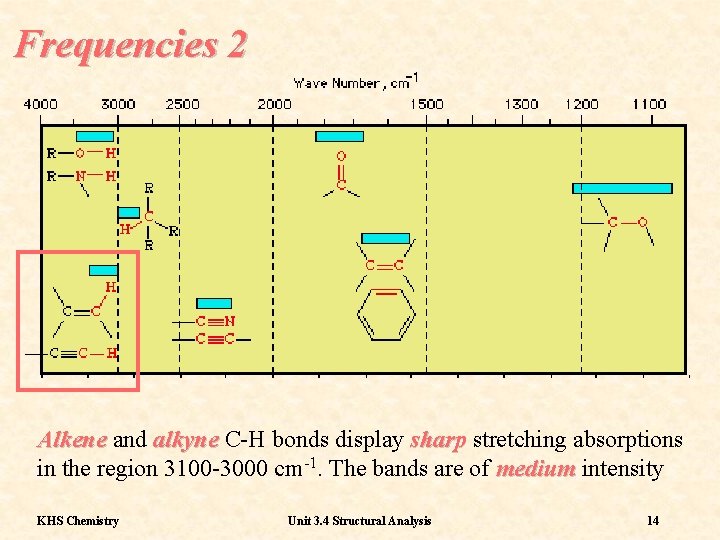
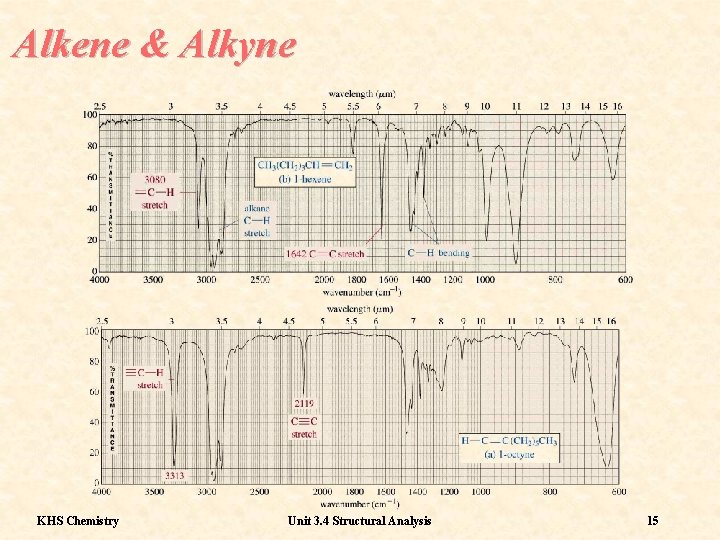
Frequencies 2 Alkene and alkyne C-H bonds display sharp stretching absorptions in the region 3100 -3000 cm-1. The bands are of medium intensity KHS Chemistry Unit 3. 4 Structural Analysis 14

Alkene & Alkyne KHS Chemistry Unit 3. 4 Structural Analysis 15

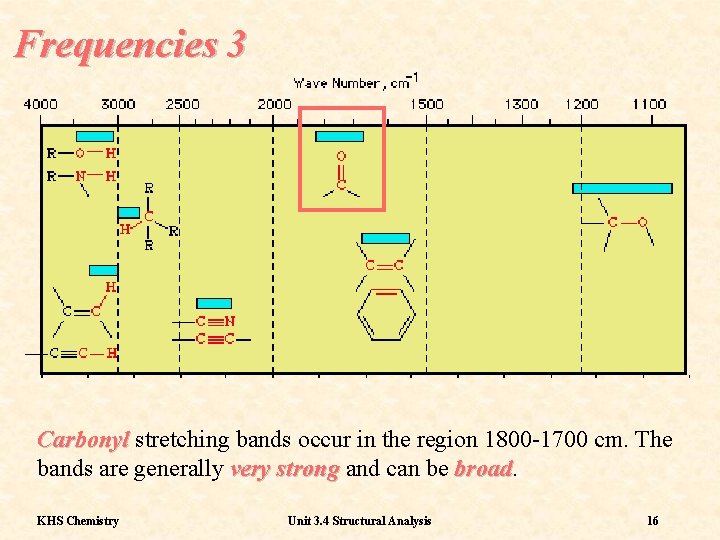
Frequencies 3 Carbonyl stretching bands occur in the region 1800 -1700 cm. The bands are generally very strong and can be broad KHS Chemistry Unit 3. 4 Structural Analysis 16

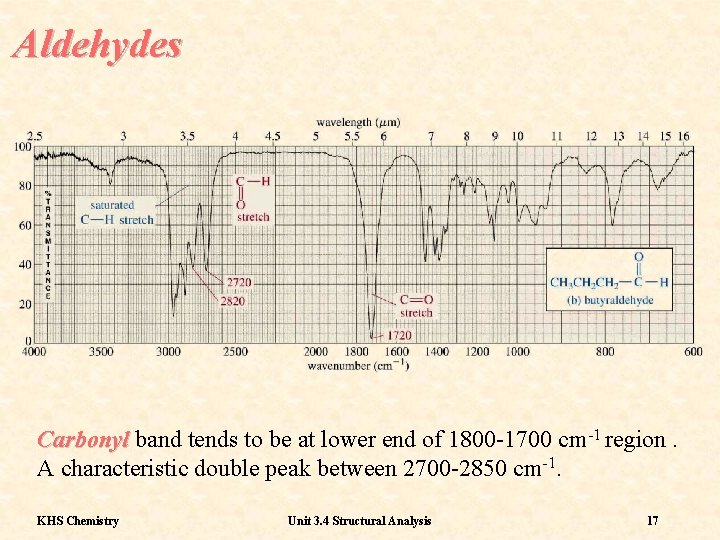
Aldehydes Carbonyl band tends to be at lower end of 1800 -1700 cm-1 region. A characteristic double peak between 2700 -2850 cm-1. KHS Chemistry Unit 3. 4 Structural Analysis 17

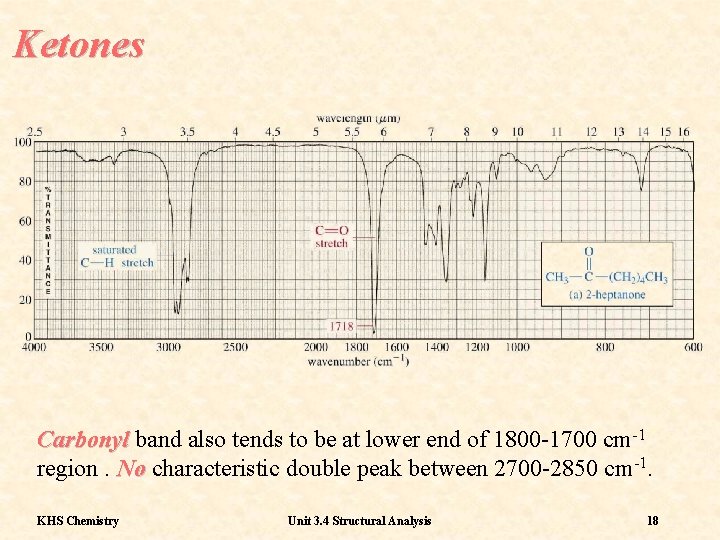
Ketones Carbonyl band also tends to be at lower end of 1800 -1700 cm-1 region. No characteristic double peak between 2700 -2850 cm-1. KHS Chemistry Unit 3. 4 Structural Analysis 18

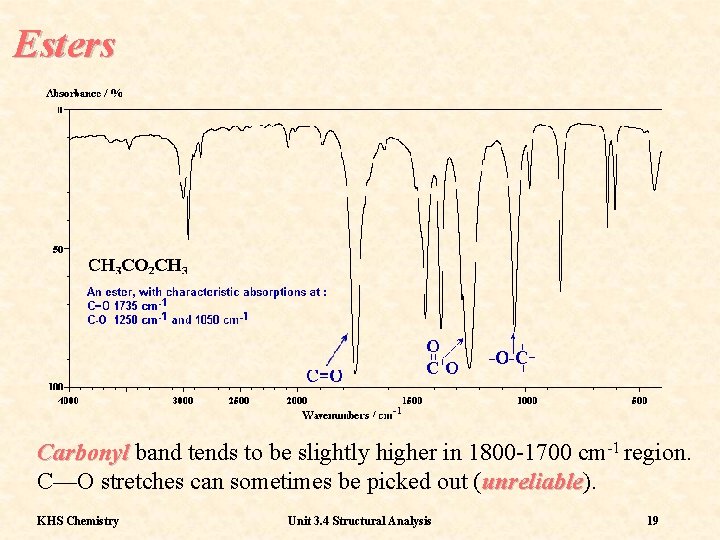
Esters Carbonyl band tends to be slightly higher in 1800 -1700 cm-1 region. C—O stretches can sometimes be picked out (unreliable). unreliable KHS Chemistry Unit 3. 4 Structural Analysis 19

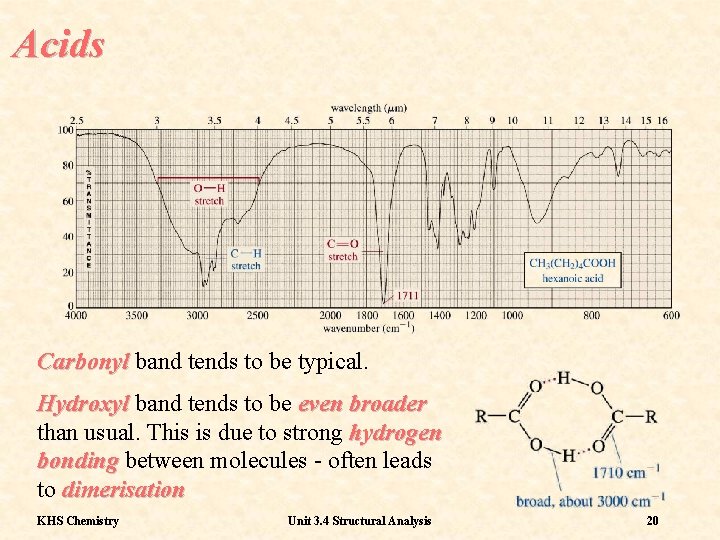
Acids Carbonyl band tends to be typical. Hydroxyl band tends to be even broader than usual. This is due to strong hydrogen bonding between molecules - often leads to dimerisation KHS Chemistry Unit 3. 4 Structural Analysis 20

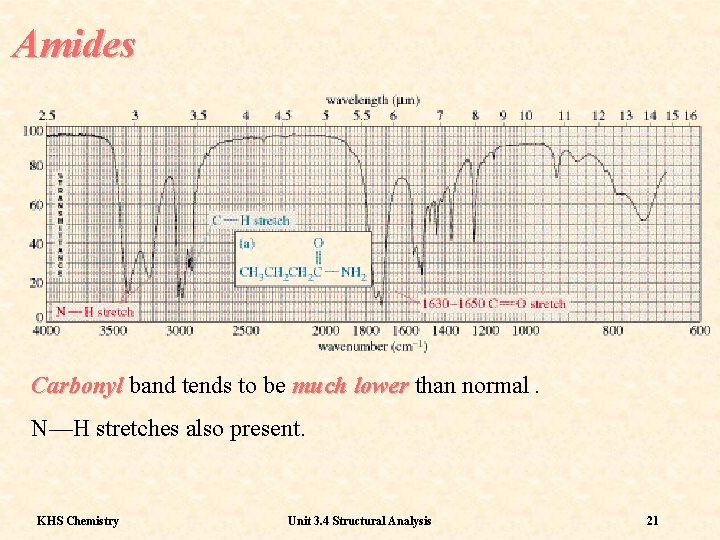
Amides Carbonyl band tends to be much lower than normal. N—H stretches also present. KHS Chemistry Unit 3. 4 Structural Analysis 21

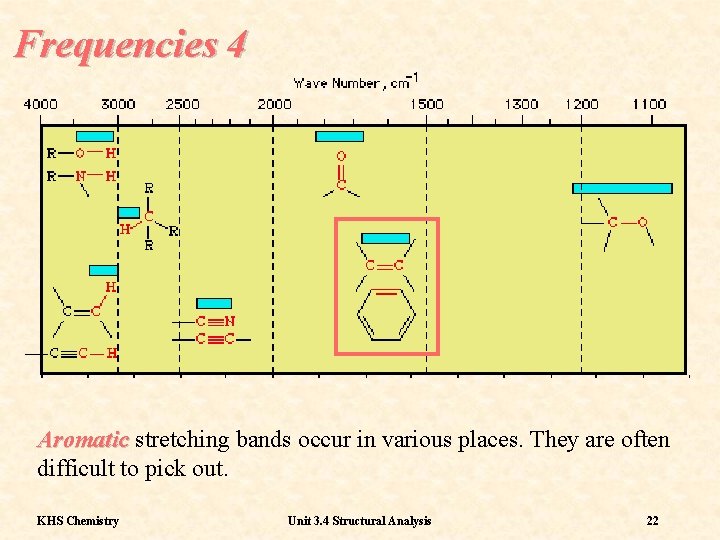
Frequencies 4 Aromatic stretching bands occur in various places. They are often difficult to pick out. KHS Chemistry Unit 3. 4 Structural Analysis 22

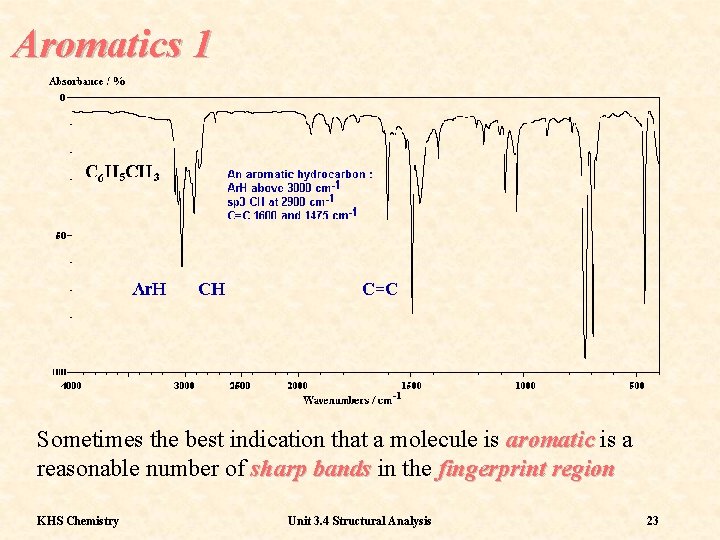
Aromatics 1 Sometimes the best indication that a molecule is aromatic is a reasonable number of sharp bands in the fingerprint region KHS Chemistry Unit 3. 4 Structural Analysis 23

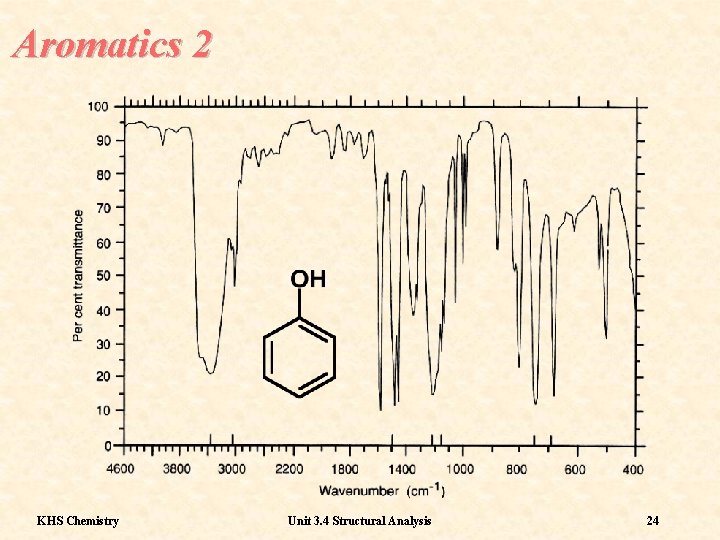
Aromatics 2 KHS Chemistry Unit 3. 4 Structural Analysis 24

Summary of IR Absorptions 1 KHS Chemistry Unit 3. 4 Structural Analysis 25

Summary of IR Absorptions 2 KHS Chemistry Unit 3. 4 Structural Analysis 26

Strengths & Limitations IR alone cannot determine a structure. Some signals may be ambiguous The functional group is usually indentifiable. The absence of a signal is definite proof that the functional group is absent. Correspondence with a known sample’s IR spectrum confirms the identity of the compound - fingerprint KHS Chemistry Unit 3. 4 Structural Analysis 27

Structural Analysis 2 End of Topic 4 KHS Chemistry Unit 3. 4 Structural Analysis 28