Electronic Structure Gordon Watson Chemistry Department Kelso High









- Slides: 33

Electronic Structure Gordon Watson Chemistry Department, Kelso High School Based on Presentations produced by David P. White University of North Carolina, Wilmington Adv Higher Unit 1 Topic 1 2/23/2021 Unit 1. 1 Electronic Structure 1

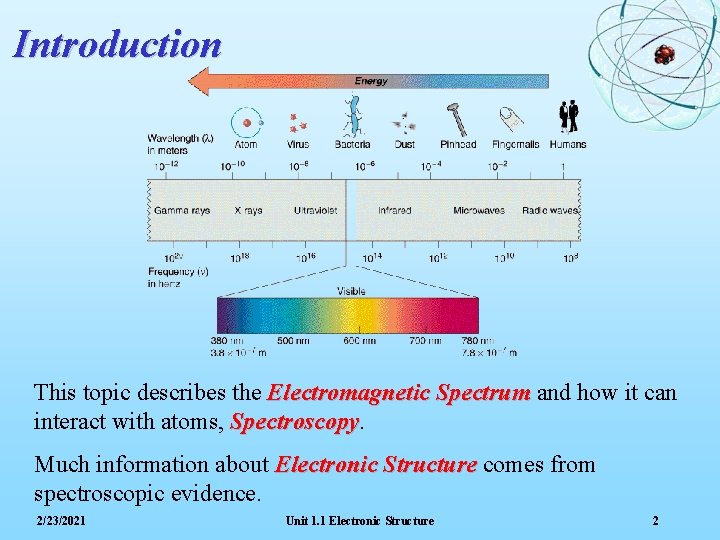
Introduction This topic describes the Electromagnetic Spectrum and how it can interact with atoms, Spectroscopy Much information about Electronic Structure comes from spectroscopic evidence. 2/23/2021 Unit 1. 1 Electronic Structure 2

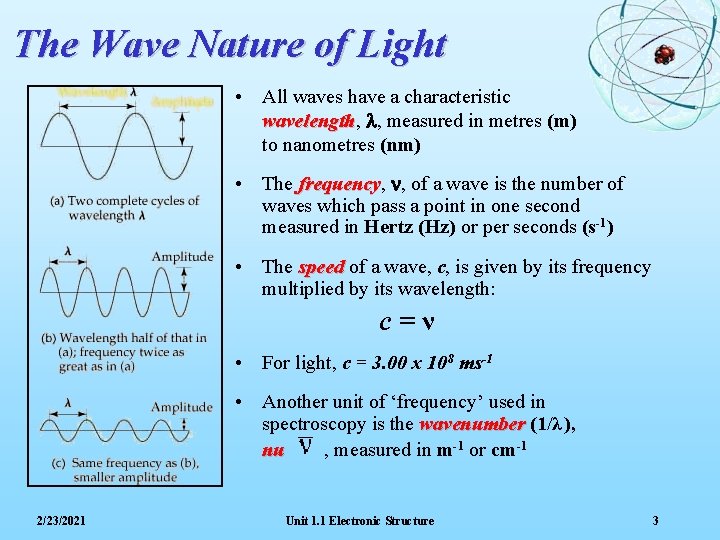
The Wave Nature of Light • All waves have a characteristic wavelength, wavelength l, measured in metres (m) to nanometres (nm) • The frequency, frequency n, of a wave is the number of waves which pass a point in one second measured in Hertz (Hz) or per seconds (s-1) • The speed of a wave, c, is given by its frequency multiplied by its wavelength: c=ν • For light, c = 3. 00 x 108 ms-1 • Another unit of ‘frequency’ used in spectroscopy is the wavenumber (1/λ ), nu , measured in m-1 or cm-1 2/23/2021 Unit 1. 1 Electronic Structure 3

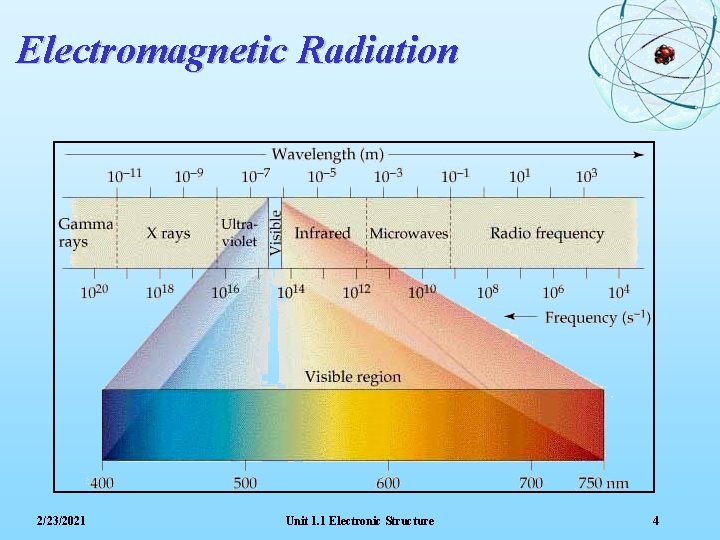
Electromagnetic Radiation 2/23/2021 Unit 1. 1 Electronic Structure 4

The Particle Nature of Light 1 Planck: Planck energy can only be absorbed or released from atoms in certain amounts called quanta To understand quantization, quantization consider the notes produced by a violin (continuous) and a piano (quantized): a violin can produce any note by placing the fingers at an appropriate spot on the fingerboard. A piano can only produce certain notes corresponding to the keys on the keyboard. 2/23/2021 Unit 1. 1 Electronic Structure 5

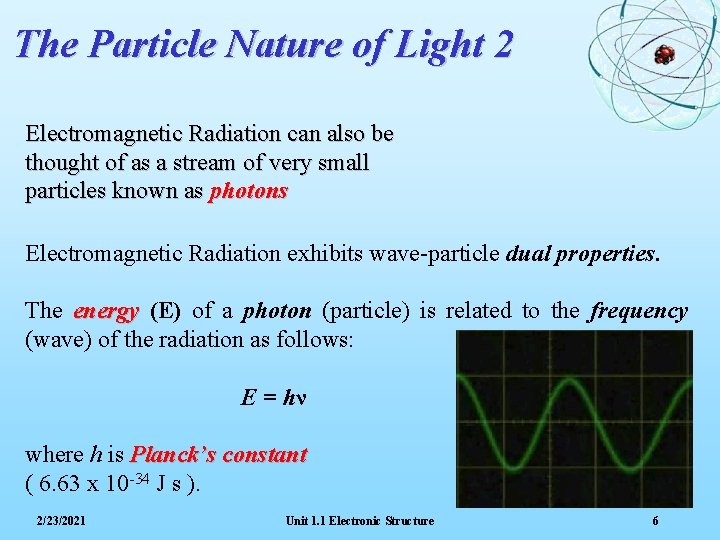
The Particle Nature of Light 2 Electromagnetic Radiation can also be thought of as a stream of very small particles known as photons Electromagnetic Radiation exhibits wave-particle dual properties. The energy (E) of a photon (particle) is related to the frequency (wave) of the radiation as follows: E = hν where h is Planck’s constant ( 6. 63 x 10 -34 J s ). 2/23/2021 Unit 1. 1 Electronic Structure 6

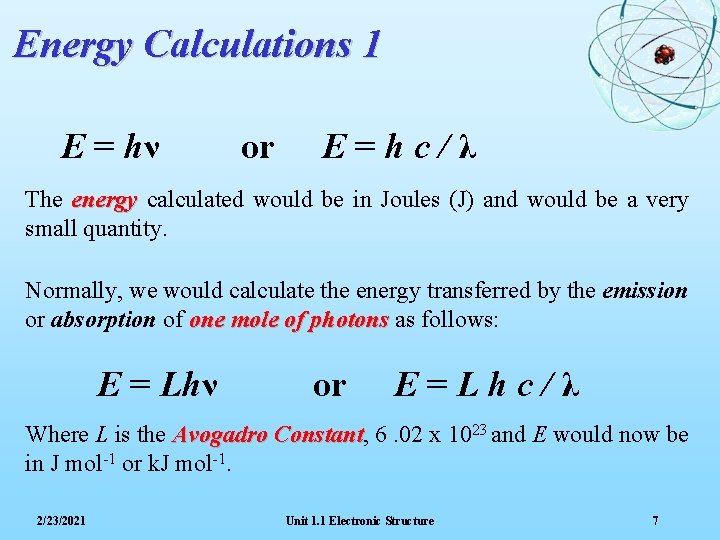
Energy Calculations 1 E = hν or E=hc/λ The energy calculated would be in Joules (J) and would be a very small quantity. Normally, we would calculate the energy transferred by the emission or absorption of one mole of photons as follows: E = Lhν or E=Lhc/λ Where L is the Avogadro Constant, Constant 6. 02 x 1023 and E would now be in J mol-1 or k. J mol-1. 2/23/2021 Unit 1. 1 Electronic Structure 7

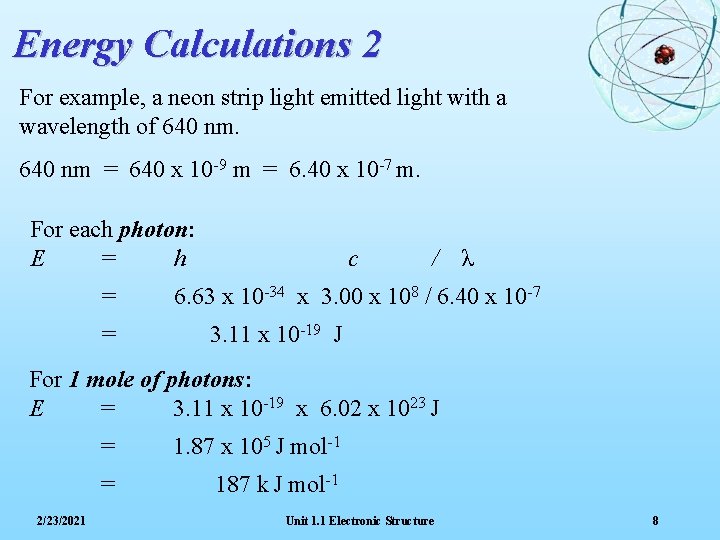
Energy Calculations 2 For example, a neon strip light emitted light with a wavelength of 640 nm = 640 x 10 -9 m = 6. 40 x 10 -7 m. For each photon: E = h = = c / λ 6. 63 x 10 -34 x 3. 00 x 108 / 6. 40 x 10 -7 3. 11 x 10 -19 J For 1 mole of photons: E = 3. 11 x 10 -19 x 6. 02 x 1023 J 2/23/2021 = 1. 87 x 105 J mol-1 = 187 k J mol-1 Unit 1. 1 Electronic Structure 8

Emission Spectra 1 Atomic emission spectra provided significant contributions to the modern picture of atomic structure Radiation composed of only one wavelength is called monochromatic Radiation that spans a whole array of different wavelengths is called continuous White light can be separated into a continuous spectrum of colors. 2/23/2021 Unit 1. 1 Electronic Structure 9

Emission Spectra 2 2/23/2021 Unit 1. 1 Electronic Structure 10

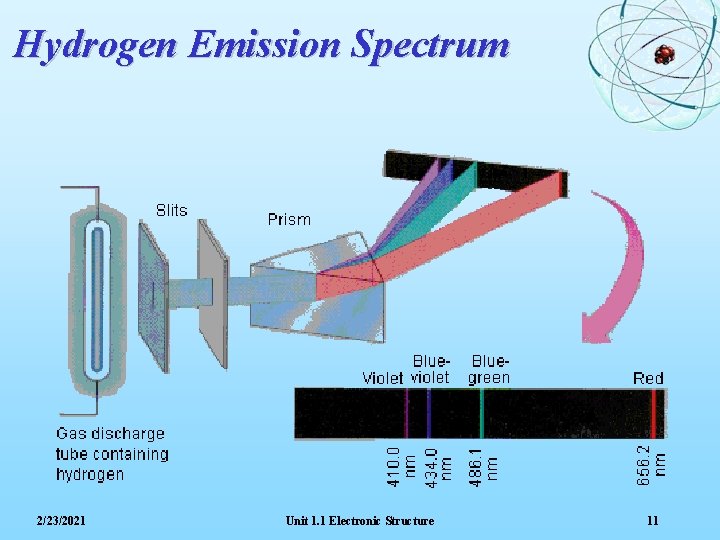
Hydrogen Emission Spectrum 2/23/2021 Unit 1. 1 Electronic Structure 11

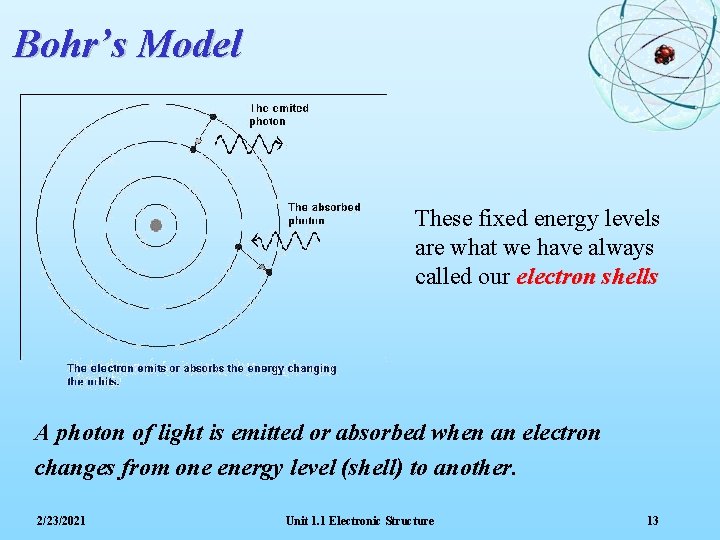
Line Spectra The spectrum obtained when hydrogen atoms are excited shows four lines: red, blue-green, blue and indigo Bohr deduced that the colours were due to the movement of electrons from a higher energy level back to the ‘ground state’. The significance of a Line Spectrum is that it suggests that electrons can only occupy certain fixed energy levels 2/23/2021 Unit 1. 1 Electronic Structure 12

Bohr’s Model These fixed energy levels are what we have always called our electron shells A photon of light is emitted or absorbed when an electron changes from one energy level (shell) to another. 2/23/2021 Unit 1. 1 Electronic Structure 13

Principal Quantum Number Bohr described each shell by a number, the principal quantum number, n For the first shell, n=1 For the second shell, n = 2 and so on. After lots of math, Bohr showed that where n is the principal quantum number (i. e. , n = 1, 2, 3, …), and RH is the Rydberg constant = 2. 18 x 10 -18 J. 2/23/2021 Unit 1. 1 Electronic Structure 14

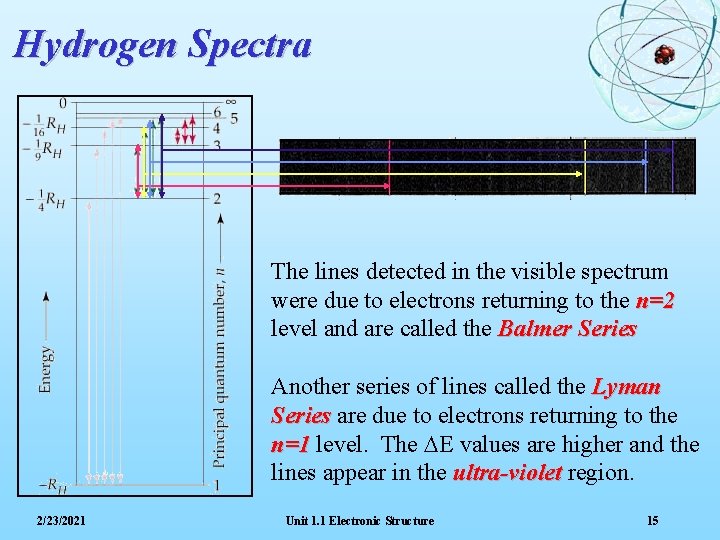
Hydrogen Spectra The lines detected in the visible spectrum were due to electrons returning to the n=2 level and are called the Balmer Series Another series of lines called the Lyman Series are due to electrons returning to the n=1 level. The DE values are higher and the lines appear in the ultra-violet region. 2/23/2021 Unit 1. 1 Electronic Structure 15

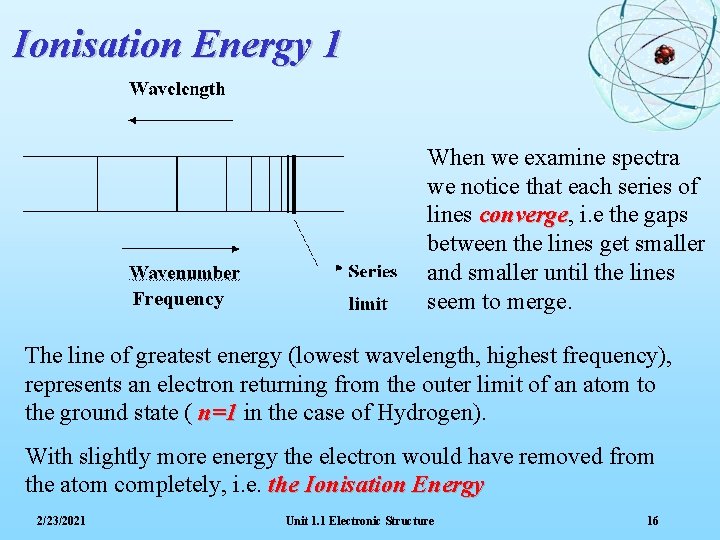
Ionisation Energy 1 Frequency When we examine spectra we notice that each series of lines converge, converge i. e the gaps between the lines get smaller and smaller until the lines seem to merge. The line of greatest energy (lowest wavelength, highest frequency), represents an electron returning from the outer limit of an atom to the ground state ( n=1 in the case of Hydrogen). With slightly more energy the electron would have removed from the atom completely, i. e. the Ionisation Energy 2/23/2021 Unit 1. 1 Electronic Structure 16

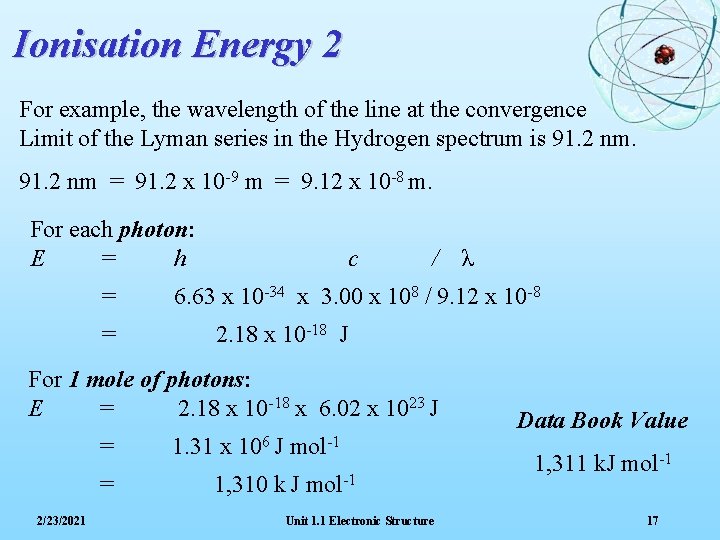
Ionisation Energy 2 For example, the wavelength of the line at the convergence Limit of the Lyman series in the Hydrogen spectrum is 91. 2 nm = 91. 2 x 10 -9 m = 9. 12 x 10 -8 m. For each photon: E = h = = c / λ 6. 63 x 10 -34 x 3. 00 x 108 / 9. 12 x 10 -8 2. 18 x 10 -18 J For 1 mole of photons: E = 2. 18 x 10 -18 x 6. 02 x 1023 J = = 2/23/2021 1. 31 x 106 J mol-1 1, 310 k J mol-1 Unit 1. 1 Electronic Structure Data Book Value 1, 311 k. J mol-1 17

Subshells - Orbitals High resolution spectra of more complex atoms reveal that lines are often split into triplets, quintuplets etc. This is evidence that Shells are further subdivided into Subshells These Subshells are called Orbitals Calculations using Quantum Mechanics have been able to determine the shapes of these Orbitals 2/23/2021 Unit 1. 1 Electronic Structure 18

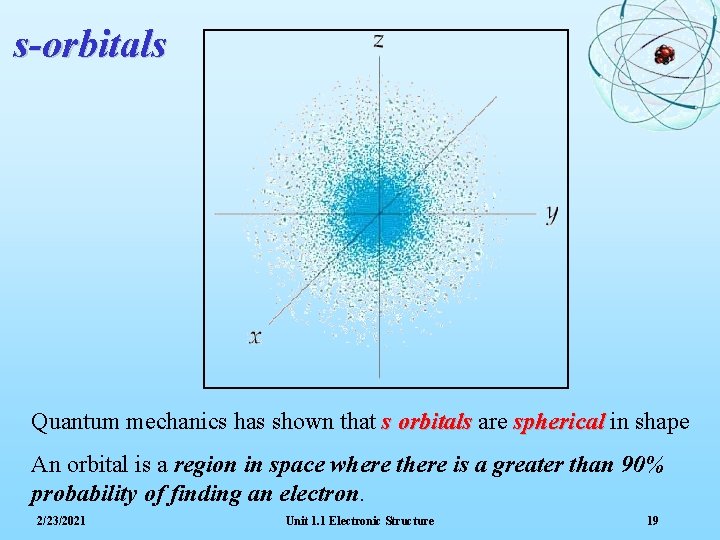
s-orbitals Quantum mechanics has shown that s orbitals are spherical in shape An orbital is a region in space where there is a greater than 90% probability of finding an electron. 2/23/2021 Unit 1. 1 Electronic Structure 19

Orbitals and Quantum Numbers Angular Quantum Number, l. This quantum number describes the shape of an orbital. l = 0, 1, 2, and 3 (4 shapes) but we use letters for l (s, p, d and f). Usually we refer to the s, p, d and f-orbitals Magnetic Quantum Number, ml. This quantum number describes the orientation of orbitals of the same shape. The magnetic quantum number has integral values between -l and +l. However, we use px , py and pz instead. There are 3 possible p -orbitals There are 5 possible d-orbitals There are 7 possible f-orbitals 2/23/2021 -2 -1 -1 Unit 1. 1 Electronic Structure 0 0 +1 +1 +2 20

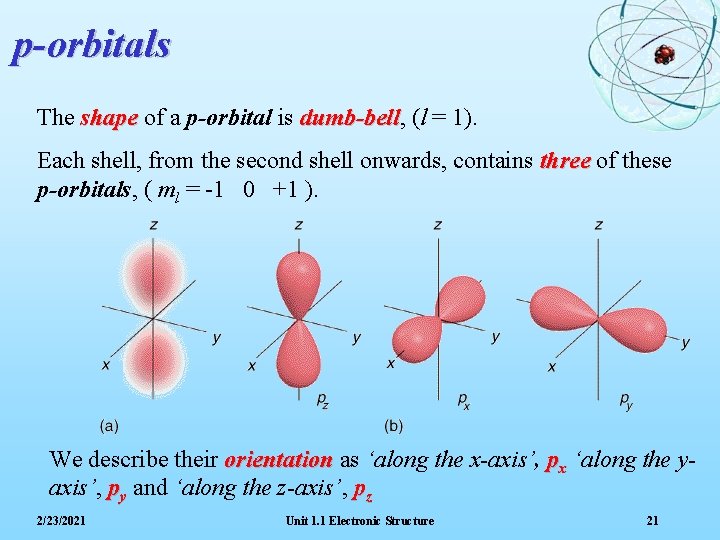
p-orbitals The shape of a p-orbital is dumb-bell, dumb-bell (l = 1). Each shell, from the second shell onwards, contains three of these p-orbitals, ( ml = -1 0 +1 ). We describe their orientation as ‘along the x-axis’, px ‘along the yaxis’, py and ‘along the z-axis’, pz 2/23/2021 Unit 1. 1 Electronic Structure 21

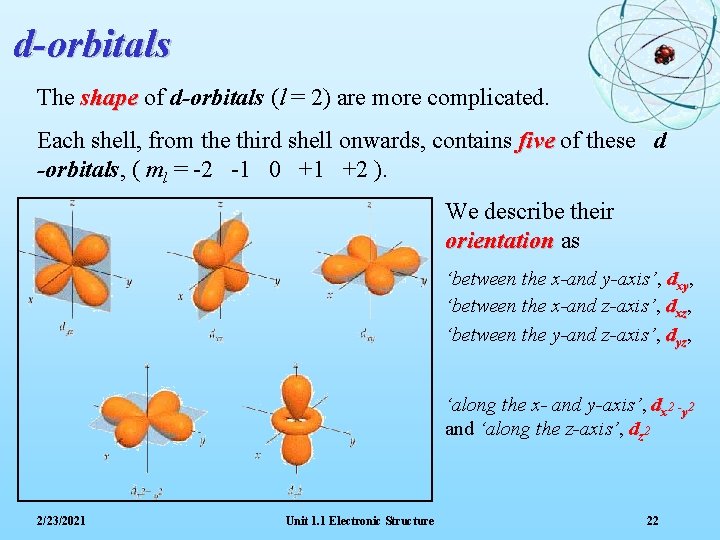
d-orbitals The shape of d-orbitals (l = 2) are more complicated. Each shell, from the third shell onwards, contains five of these d -orbitals, ( ml = -2 -1 0 +1 +2 ). We describe their orientation as ‘between the x-and y-axis’, dxy, ‘between the x-and z-axis’, dxz, ‘between the y-and z-axis’, dyz, ‘along the x- and y-axis’, dx 2 -y 2 and ‘along the z-axis’, dz 2 2/23/2021 Unit 1. 1 Electronic Structure 22

f-orbitals The shape of f-orbitals (l = 3) are even more complicated. Each shell, from the fourth shell onwards, contains seven of these f-orbitals, ( ml = -3 -2 -1 0 +1 +2 +3 ). f-orbitals are not included in Advanced Higher so we will not have to consider their shapes or orientations, thank goodness! 2/23/2021 Unit 1. 1 Electronic Structure 23

f-orbitals Couldn’t resist it ! 2/23/2021 Unit 1. 1 Electronic Structure 24

Spin Quantum Number Each orbital can hold up to 2 electrons. In 1920 it was realised that an electron behaves as if it has a spin A fourth quantum number was needed. The spin quantum number, number ms only has two values +1/2 and - 1/2 Therefore, up to four quantum numbers, numbers n (shell), l (shape), ml (orientation) and ms (spin) are needed to uniquely describe every electron in an atom. 2/23/2021 Unit 1. 1 Electronic Structure 25

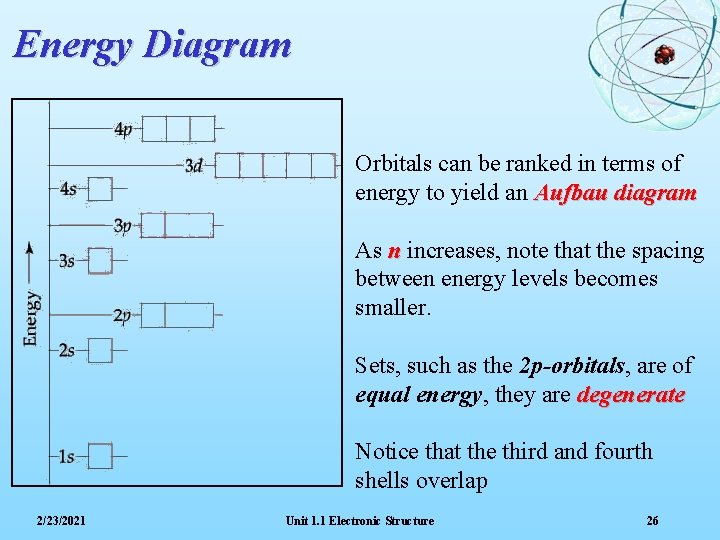
Energy Diagram Orbitals can be ranked in terms of energy to yield an Aufbau diagram As n increases, note that the spacing between energy levels becomes smaller. Sets, such as the 2 p-orbitals, are of equal energy, they are degenerate Notice that the third and fourth shells overlap 2/23/2021 Unit 1. 1 Electronic Structure 26

Electron Configurations 1 There are 3 rules which determine in which orbitals the electrons of an element are located. The Aufbau Principle states that electrons will fill orbitals starting with the orbital of lowest energy. For degenerate orbitals, electrons fill each orbital singly before any orbital gets a second electron (Hund’s Rule of Maximum Multiplicity). Multiplicity The Pauli Exclusion Principle states that the maximum number of electrons in any atomic orbital is two……. . and …. . if there are two electrons in an orbital they must have opposite spins (rather than parallel spins). 2/23/2021 Unit 1. 1 Electronic Structure 27

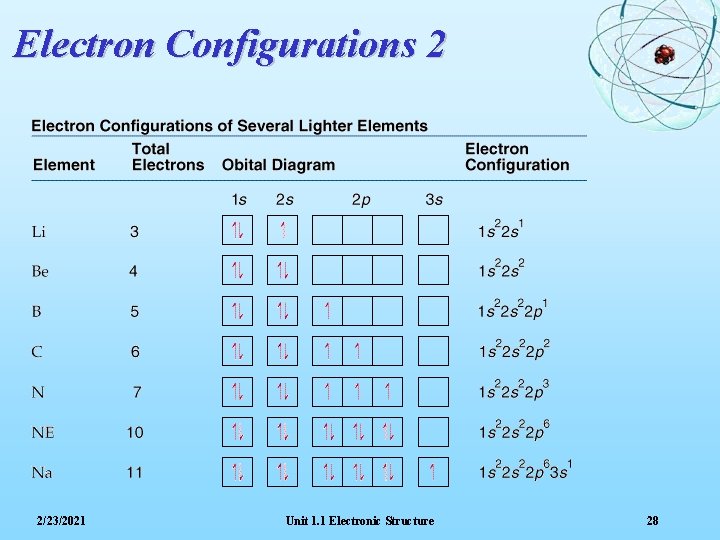
Electron Configurations 2 2/23/2021 Unit 1. 1 Electronic Structure 28

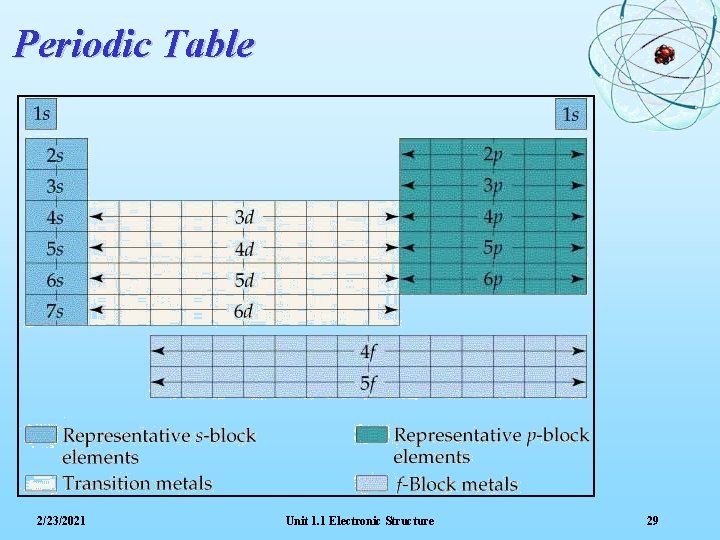
Periodic Table 2/23/2021 Unit 1. 1 Electronic Structure 29

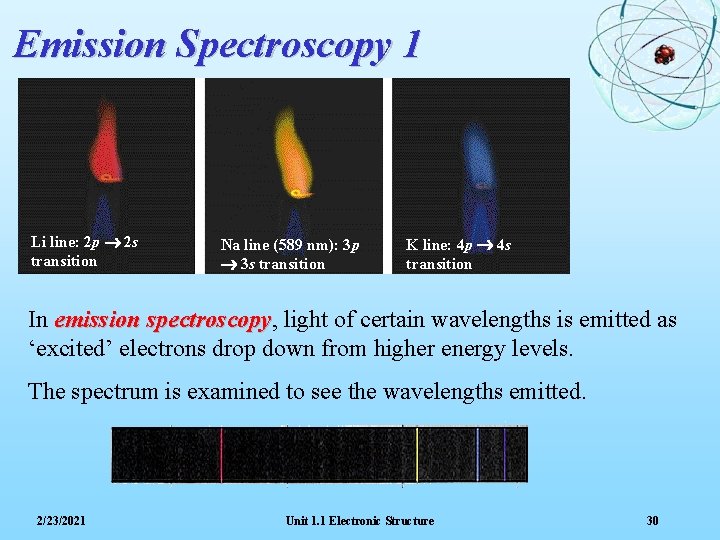
Emission Spectroscopy 1 Li line: 2 p 2 s transition Na line (589 nm): 3 p 3 s transition K line: 4 p 4 s transition In emission spectroscopy, spectroscopy light of certain wavelengths is emitted as ‘excited’ electrons drop down from higher energy levels. The spectrum is examined to see the wavelengths emitted. 2/23/2021 Unit 1. 1 Electronic Structure 30

Emission Spectroscopy 2 Each element provides a characteristic spectrum which can be used to identify the element. Analysing light from stars etc, tell us a lot about the elements present. The intensity of a particularly strong line in an element’s spectrum can be measured. The intensity of the light emitted is proportional to the quantity of the atoms/ions in the sample. Calibration samples can be prepared, intensities measured and unknown concentrations determined. Analytical tool 2/23/2021 Unit 1. 1 Electronic Structure 31

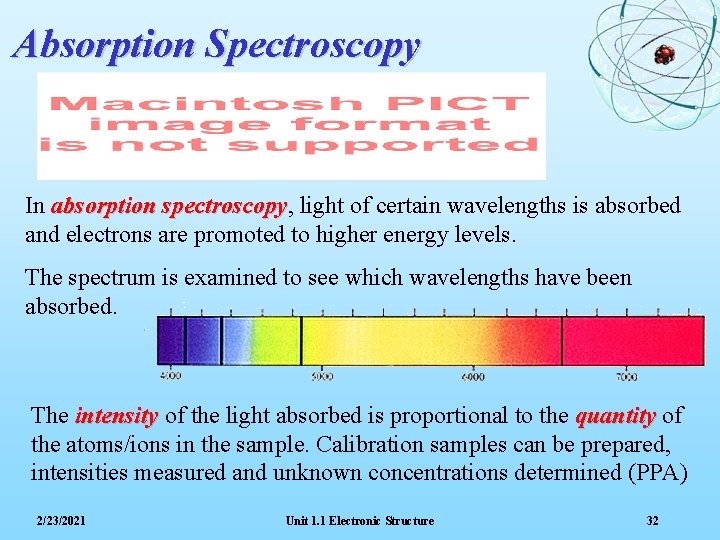
Absorption Spectroscopy In absorption spectroscopy, spectroscopy light of certain wavelengths is absorbed and electrons are promoted to higher energy levels. The spectrum is examined to see which wavelengths have been absorbed. The intensity of the light absorbed is proportional to the quantity of the atoms/ions in the sample. Calibration samples can be prepared, intensities measured and unknown concentrations determined (PPA) 2/23/2021 Unit 1. 1 Electronic Structure 32

Electronic Structure of Atoms End of Topic 1 2/23/2021 Unit 1. 1 Electronic Structure 33
 Kelso high school chemistry
Kelso high school chemistry Gordon kelso chemistry
Gordon kelso chemistry Kelso high school chemistry
Kelso high school chemistry Gordon watson ap chemistry
Gordon watson ap chemistry Gordon watson chemistry
Gordon watson chemistry Kelso high school chemistry
Kelso high school chemistry Gordon watson chemistry
Gordon watson chemistry John b. watson james broadus watson
John b. watson james broadus watson John broadus watson emma watson
John broadus watson emma watson John b watson conductismo
John b watson conductismo Mary ickes watson
Mary ickes watson Ap chemistry electronic structure of atoms
Ap chemistry electronic structure of atoms Is the electronic exchange of money or scrip
Is the electronic exchange of money or scrip Electronic news gathering and electronic field production
Electronic news gathering and electronic field production Kelso's choices
Kelso's choices Ts kelso
Ts kelso Central welding supply kelso wa
Central welding supply kelso wa Bill frakes
Bill frakes What is electronic configuration
What is electronic configuration Usf chemistry
Usf chemistry Nit calicut chemistry department
Nit calicut chemistry department Manipal university chemistry department
Manipal university chemistry department Texas tech chemistry department
Texas tech chemistry department Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Halogens electron structure
Halogens electron structure Pn junction band diagram
Pn junction band diagram Chapter 6 electronic structure of atoms answers
Chapter 6 electronic structure of atoms answers Quantum theory and the electronic structure of atoms
Quantum theory and the electronic structure of atoms Which of the d orbitals most resembles a pz orbital?
Which of the d orbitals most resembles a pz orbital? How to write a parody song
How to write a parody song Techniques for avoiding resource overload? *
Techniques for avoiding resource overload? * Human resource management lecture chapter 1
Human resource management lecture chapter 1 Organizational structure of finance department
Organizational structure of finance department