Storage of Samples Nandini K Kumar Institute of






















- Slides: 33

Storage of Samples Nandini K. Kumar Institute of Institute, Chennai nandkku@gmail. com

Source of samples • Stored samples • • Left over samples after clinical investigations Left over samples from research project Autopsy specimens Embryo and Foetal tissue Tissue banks including cord blood banks Wastes including abandoned tissue Donor samples Collection for prospective study

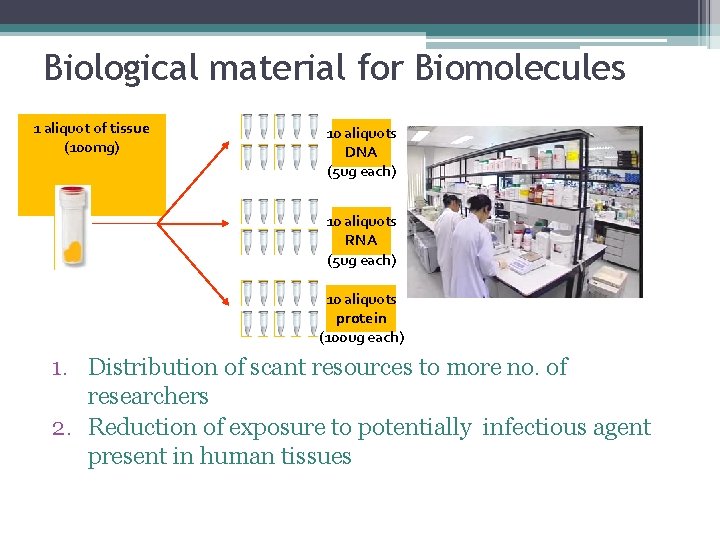
Biological material for Biomolecules 1 aliquot of tissue (100 mg) 10 aliquots DNA (5 ug each) 10 aliquots RNA (5 ug each) 10 aliquots protein (100 ug each) 1. Distribution of scant resources to more no. of researchers 2. Reduction of exposure to potentially infectious agent present in human tissues

Core Elements of Research Review involving Human Tissues • • • Nature of research Intended use of samples Length of conservation Secondary uses Legal context Specific concerns raised by researcher, ethics committees & sponsors • Selection of greatest degree of privacy protection compatible with the objectives of the research Y Joly, BM Knoppers & MT Nguyen. Stored Tissue Samples: through the confidentiality maze. The Pharmacogenomics Journal Nature 2005

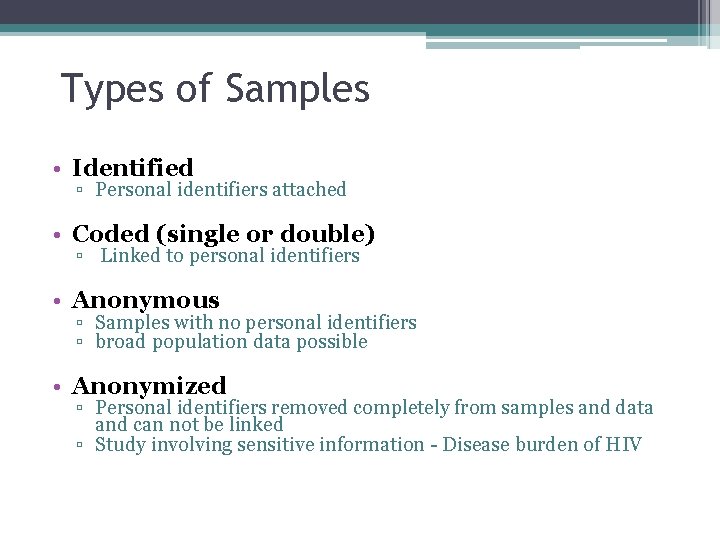
Types of Samples • Identified ▫ Personal identifiers attached • Coded (single or double) ▫ Linked to personal identifiers • Anonymous ▫ Samples with no personal identifiers ▫ broad population data possible • Anonymized ▫ Personal identifiers removed completely from samples and data and can not be linked ▫ Study involving sensitive information - Disease burden of HIV

National Pathology Repository - US • 22 subspecialty departments with a combined workforce of over 820 personnel, including over 120 pathologists and other scientists • > 2. 8 m cases accessioned and coded since 1917 located at AFIP. • Written records and >50 m microscopic slides, 30 m paraffin tissue blocks, and 12 m preserved wet tissue specimens. • Annually, approximately 60, 000 new cases are accessioned and coded from both sexes, all races/ethnicities, all ages, and come from contributors worldwide. • Represent the entire spectrum of human and animal disease. • Stores case files and specimens from over 20 closed military medical facilities. • Currently being inventoried and digitized and will potentially be made available to the public for research and educational efforts. • AFIP has contracted for a marketing survey that is in the process of being conducted to determine how best to publicize and make use of this material.

7 The “Simplest” Repository Model Informed Consent? Repository Policies Human Subjects? IRB Review? Privacy requirements? State, local, inst. requirements? International regulations? Ethical/responsible research Informed Consent?

Biobank Governance

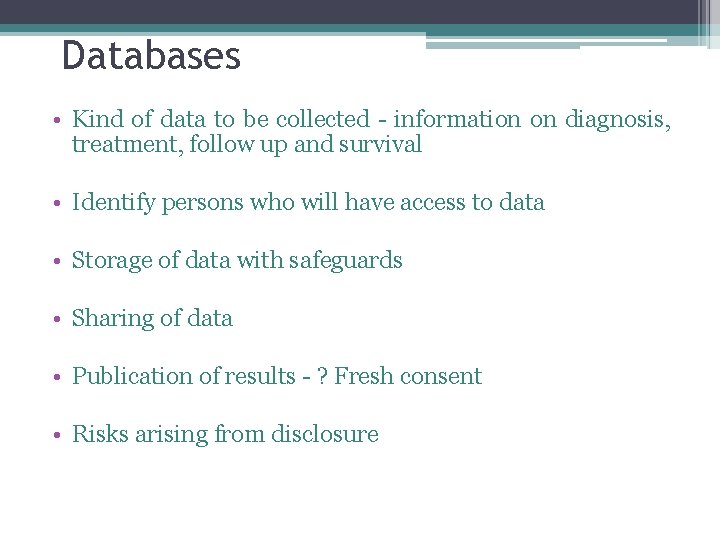
Databases • Kind of data to be collected - information on diagnosis, treatment, follow up and survival • Identify persons who will have access to data • Storage of data with safeguards • Sharing of data • Publication of results - ? Fresh consent • Risks arising from disclosure

Benefits • Improvement in knowledge/ Science • Medical benefit for Individual - present vs future • Diagnostics and drug/ therapy for population • Pharmacogenomic studies - information predispositions and response to drug about • Benefit sharing : Commercial value through Patents

Fears • Uncertain clinical significance – insurance, legal, loss of job / admissions • Unwanted informational flow – psychosocial risk : Anxiety, Depression, Stigma, Discrimination on breach of confidentiality • Blood samples taken for some hidden purpose such as cloning or satanic rituals. • Not appropriate or socially acceptable to take some types of samples (e. g. , vaginal swabs from unmarried women or stool samples). • People may not want to provide a sample / get results if the test is for a disease with no cure. • Transport out of the country – regulatory issue

Informed Consent Issues • Risk dependent ? – different consent standards • Storage & links • Sample sharing by researchers • Samples from deceased or non-traceable persons • Benefit sharing in commercialisation • Right to information




Special issues • Computerisation of Patient record systems • Physicians employed by private organisations/ in defense institutions • Genetic or Mother to child infectious transmissions – transmission guilt

Broad / blanket consent • When human biological materials are collected, whether in a research or clinical setting, it is appropriate to ask subjects for their consent to future use of their samples, even when such uses are at the time unknown. NBAC (1999) p. 63 • Participants should be informed of possibility of future uses of data, beyond the limits of present consent, and should be provided with an opportunity to withhold consent to such uses WHO (2003) rec. 6(8) • Informed consent may include blanket consent HUGO (2002) rec. 4(a) • A reasonable policy is to secure a degree of flexibility by allowing the subject to consent to a range of related studies over time Consortium on Pharmacogenetics (2002) Research B

Waiver of consent • No more than minimal risk to subjects • Does not adversely affect rights and welfare of subjects • Research could not practicably be carried out without waiver • Whenever appropriate, subjects will be provided with additional pertinent information after participation NBAC (1999) p. 66; See also: AMA (2003); TCPS (1998)

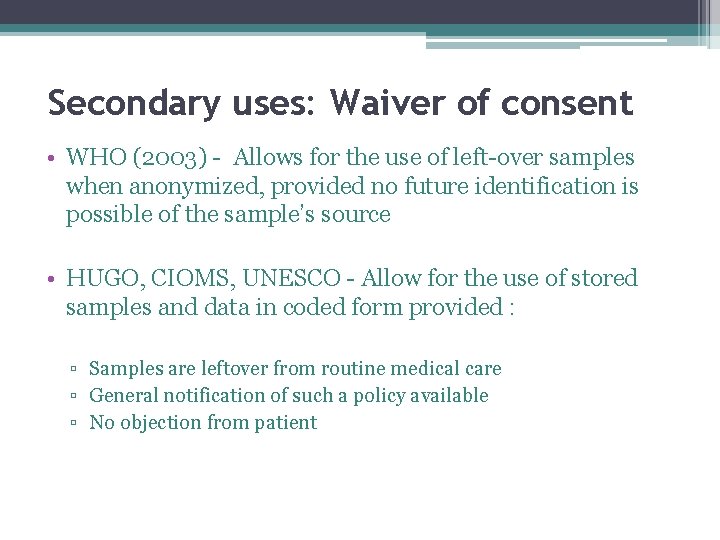
Secondary uses: Waiver of consent • WHO (2003) - Allows for the use of left-over samples when anonymized, provided no future identification is possible of the sample’s source • HUGO, CIOMS, UNESCO - Allow for the use of stored samples and data in coded form provided : ▫ Samples are leftover from routine medical care ▫ General notification of such a policy available ▫ No objection from patient

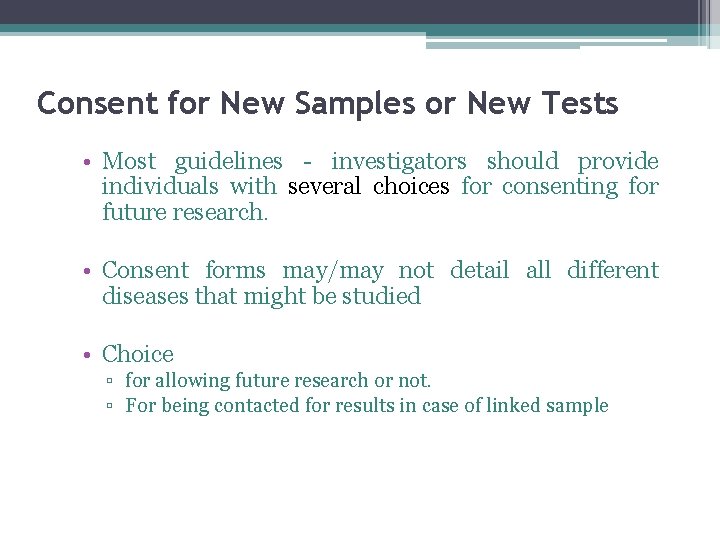
Consent for New Samples or New Tests • Most guidelines - investigators should provide individuals with several choices for consenting for future research. • Consent forms may/may not detail all different diseases that might be studied • Choice ▫ for allowing future research or not. ▫ For being contacted for results in case of linked sample

Ethics Committee Considerations • Whether information can be obtained in a manner that allows individuals to consent; • Whether proposed research is scientifically sound; • How difficult it would be to re-contact subjects; • How the availability of effective medical interventions affects appropriateness of pursuing anonymous research • Community consultation whenever applicable

John Moore Case Study • Spleen cancer patient • Dr Golde, kept cells from his cancerous spleen alive in a lab after Moore's surgery and did not tell him. • Dr. Golde made Moore come many times for follow - up • After 7 yrs, Moore realized the doctor had patented the cells and sold them for stock worth $1. 5 million USD • Moore sued Dr. Golde and lost on legal ownership issue.

Biological Samples • Researchers to Return Blood Samples to the Yanomamö tribe of Amazon of S. America • Rebecca Skloot, science writer and author of “The Immortal Life of Henrietta Lacks” ▫ Hela cell line • Samples of Havasupai Tribe in Arizona returned

Indian Guidelines, Rules & Regulations • Indian Guidelines – ICMR, DBT • Drugs Controller General of India’s Office, Drugs & Cosmetics Act, 1940 Schedule Y amendment Jan. 2005 • Health Ministry’s Screening Committee • District Magistrate’s permission • Draft Human Genome Regulatory Bill

Draft Human Genome Regulatory Bill • To document human genetic samples; • Control access of the samples for research purposes; • Ensure commercial benefits derived from the research are shared especially with the community that lends the samples; • Possible establishment of a repository for human genetic samples which allow for hospitals and laboratories affiliated with the programme to lend research samples; • Also smaller tribes, especially those with unbroken genetic lines, can provide samples to the respository which will preserve the same as a Bank.

DNA Banking - ICMR Guidelines (2006) Primary Use approved by IEC i. Original intent for which consent is taken ii. Right to withdraw any time iii. if sample is inadequate or contaminated re-contact is necessary for fresh and specific consent to be incorporated in the prior consent form; iv. while obtaining data/samples from vulnerable subgroups with reduced autonomy, informed consent to be obtained from legally authorized representatives in the presence of impartial witness. The risks and benefits should be adequately explained;

Primary Use (contd. ) v. when samples are from specified communities, permission of the group leader/local leader/authorities must also be obtained. However individual consent should never be compromised even if permission of the gatekeepers/village panchayat vi. group consent of the population/community should be obtained through its culturally appropriate authorities before sampling starts, particularly so for group specific research like genetic research; vii. samples obtained for archival purposes in a prospective study.

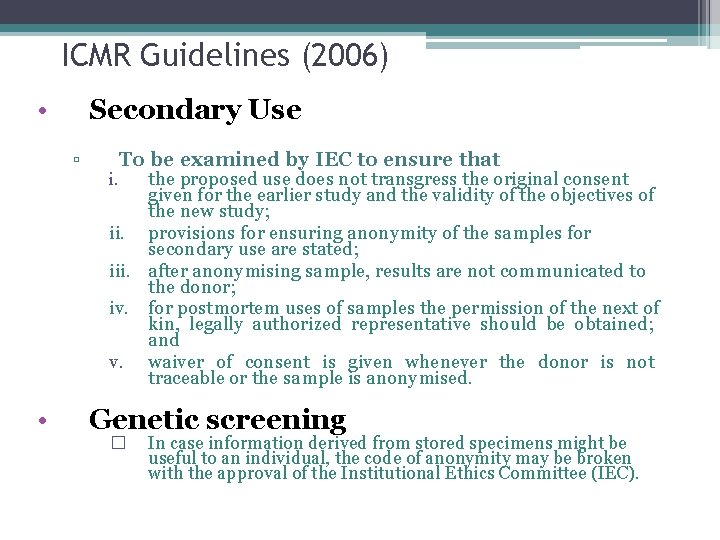
ICMR Guidelines (2006) • Secondary Use ▫ • To be examined by IEC to ensure that the proposed use does not transgress the original consent given for the earlier study and the validity of the objectives of the new study; ii. provisions for ensuring anonymity of the samples for secondary use are stated; iii. after anonymising sample, results are not communicated to the donor; iv. for postmortem uses of samples the permission of the next of kin, legally authorized representative should be obtained; and v. waiver of consent is given whenever the donor is not traceable or the sample is anonymised. i. Genetic screening � In case information derived from stored specimens might be useful to an individual, the code of anonymity may be broken with the approval of the Institutional Ethics Committee (IEC).

Donors Commercial Interests Scientists Medical Applications

Earlier “Take & run” mode “Biopiracy”

Transfer of Biological Material • DNA samples analysis by Indian scientists/ laboratories. • No sample to be sent out without following GOI guidelines • Failure of agreement - guidelines of the country (India) shall prevail. • International collaboration - well-documented MOU, approved by IEC ▫ Scope of utilization of exchanged material and related IPR issues ▫ Concerns for human rights ▫ Scientists involved shall ensure : �Consent for collection of samples �Access to these samples and for what purposes �Property rights of the DNA �Quality control of the laboratories shall be appropriately documented in the research proposal for scrutiny by the IEC Major concern - generated information may produce ethnic disharmony.

Transfer of Biological Material • Review mechanism of international collaborative research ▫ IEC Clearance ▫ PRC ▫ HMSC – High Level Committee • MTA • DGFT role • District Magistrate’s approval – tribal studies

Does our DNA compel us to seek higher power, believe it or not, some scientists say yes Plus: Plus A quiz. How spiritual are you? Thank You
 Medical school in south africa
Medical school in south africa Nandini name signature
Nandini name signature Dr adarsh rajendran
Dr adarsh rajendran Uses rigid metallic platters
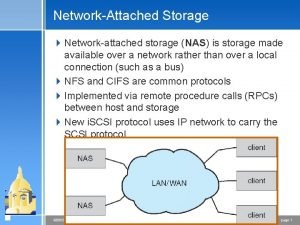
Uses rigid metallic platters Object based and unified storage
Object based and unified storage Secondary storage vs primary storage
Secondary storage vs primary storage Primary storage and secondary storage
Primary storage and secondary storage Testing the difference between two means dependent samples
Testing the difference between two means dependent samples Paper presentation samples
Paper presentation samples Launchpad rcboe login
Launchpad rcboe login Math portfolio examples
Math portfolio examples Noting and drafting samples
Noting and drafting samples Persuaive words
Persuaive words Islamic studies lesson plan samples
Islamic studies lesson plan samples Imaginative composition samples
Imaginative composition samples Matched sample design
Matched sample design Sample perspective drawing
Sample perspective drawing Telpas writing samples 2020
Telpas writing samples 2020 How to start opinion paragraph
How to start opinion paragraph Toeic speaking questions 4-6 samples
Toeic speaking questions 4-6 samples Types of water samples
Types of water samples Formula for t test independent samples
Formula for t test independent samples Ap stats chapter 24 paired samples and blocks
Ap stats chapter 24 paired samples and blocks Eal pathways
Eal pathways Ess ia marking criteria
Ess ia marking criteria Concentric tube thief is used for
Concentric tube thief is used for Batik samples
Batik samples Saskreads
Saskreads Lube samples bulk
Lube samples bulk Food inspectors seize a minimum of ___ samples a month
Food inspectors seize a minimum of ___ samples a month Log book entries example
Log book entries example Example of successive independent samples design
Example of successive independent samples design Definition essay sample
Definition essay sample Tda rubric south carolina
Tda rubric south carolina