Collection and preservation of water samples COLLECTION AND

























- Slides: 41

Collection and preservation of water samples

COLLECTION AND PRESERVATION OF WATER SAMPLES • Integral part of the total analytical procedure • The amount of water sample collected from pond should be a true representative of the water body under investigation • Evaluation of water quality will be carried out for collection of water samples, preservation, analysis and interpretation

OBJECTIVES OF THE COLLECTION OF SAMPLES • To collect representative sample in suitable volume for analysis • To maintain water properties original to the sample • To keep the concentration of all components as in the original sample • To transport sample with no significant changes in the composition of water

PRECAUTIONS • Collect samples in cleaned water bottles • Handle with care so that the sample should not deteriorate • Before filling the bottle rinse the bottle 2 or 3 times with the water to be sampled • Keep air space in the bottle

• Sample bottle should be properly stoppard • Each sample must be treated individually with regard to the substances to be determined • Make record of every sample collected and identify each bottle by attaching an appropriate inscribed tag or label-date- name, date, sampler, location, water temperature, water level, weather conditions etc.

i) River / stream /estuaries sample • Collect sample from upper reaches and lower reaches and mix to get composite sample • In estuaries collect sample following high and low tide ii) Ground water or well water • Pump sufficient water before collecting the representative ground water • Well water – collect sample using standard water samplers • Surface water by using clean bucket

iii). Lake or reservoirs • Surface sample – Bucket • Substance sample - Using proper water bottle • Near bottom sample - Horizontal water samplers • From selected location, depth, outlet and purpose, investigation

Types of water sample • Catch water sample • Composite sample • Integrated sample Catch water sample • As the source of water is known to vary with time, the water samples collected at different time intervals and analyzed separately can be of great value • Choose sampling intervals and duration on the basis of changes that occur over short or long, residual, biodegradable etc

Composite sample • It is the mixture of samples collected at the sampling points at different time intervals especially in estuarine waters Integrated samples • It is the mixture of samples collected from different points simultaneously

Types of water samplers i) Bucket ii) Vandorns sampler iii) Water bottle sampler – Nansen water bottle, NIO sampler, Niskin water bottle, Universal water sample. etc iv) Dussarts water sampler v) Horizontal water samplers Sampler containers • Plastic • Glass Nansen water bottle

Quantity of sample • Sufficient sample for analysis – 2 liters or larger volume of sample for pigments, TSS, TDS, metals Sample preservation • The preservatives will help to retard the chemical and biological changes during storage for short duration • Parameters - temperature, p. H alter significantly and the concentrations of dissolved gases such as DO, CO 2 etc. greatly affected • Better to determine the temperature, p. H, dissolved gasses in the field immediately soon after the collection. Note: • Immediate analysis is ideal for better results • Storage at low temperature is the best • Use chemical preservatives only when they do not interfere with analysis

Preservation methods i) p. H and temperature control ii) Chemical addition iii) Refrigeration iv) Freezing

MEASUREMENT OF PHYSICAL AND CHEMICAL WATER PARAMETERS • In fish culture, water quality is usually defined as the suitability of water for survival and growth of fishers and normally governed by physical, chemical and biological characteristics of water and soil (Boyd, 1978) PHYSICAL PARAMETERS • Temperature • Transparency • Turbidity • Hydrogen in concentration • Electrical Conductivity • Salinity • Chlorinity • Total solids – TDS, TSS, TVDS

CHEMICAL PARAMETERS • Dissolved oxygen • Free Carbon dioxide • Total Alkalinity • Total Hardness MAJOR IONS • Calcium • Magnesium NUTRIENTS • Inorganic nitrogen • Inorganic phosphorous • Silicate • Carbon

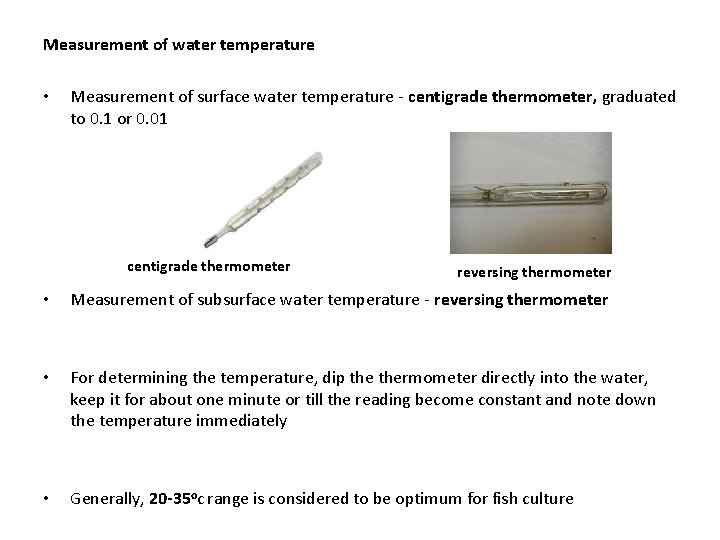
Measurement of water temperature • Measurement of surface water temperature - centigrade thermometer, graduated to 0. 1 or 0. 01 centigrade thermometer reversing thermometer • Measurement of subsurface water temperature - reversing thermometer • For determining the temperature, dip thermometer directly into the water, keep it for about one minute or till the reading become constant and note down the temperature immediately • Generally, 20 -35 oc range is considered to be optimum for fish culture

• Turbidity - measure of the interference caused due to suspended matter like clay, silt, inorganic materials such and organic materials such as phytoplankton and other microorganisms Measured by: • Jackson Turbidity Meter • Nephalometer • Take a well mixed sample in a Nephelometer sample tube and find out the value on scale following the procedure • Calculation: Turbidity (NTU) = Nephelmeter reading X 0. 4 X dilution factor

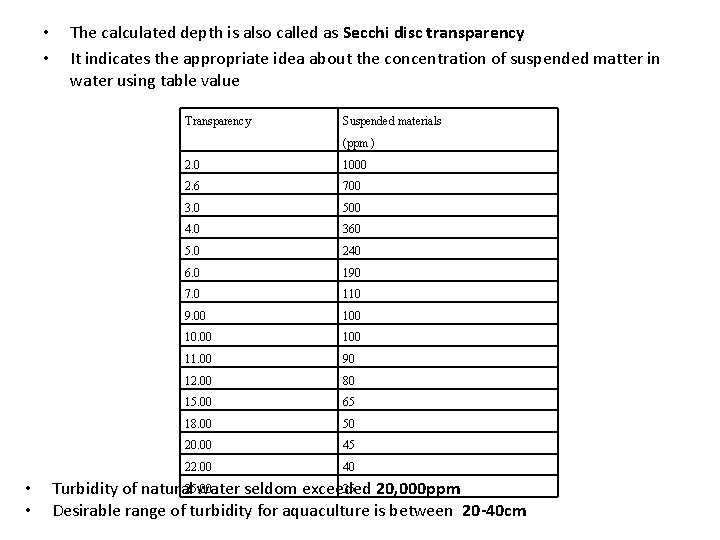
Secchi disc: • Sacchi disk - used to measure light penetration or transparency of light in water • A standard Secchi disc (10 cm. dia. ) is gradually lowered into the water to a depth at which the upper surface of the disc just disappears is noted (D 1) • The disc is lifted upward slowly and the depth at which it reappears is noted (D 2) • The sacchi disc depth or light illumination depth • Roughly it indicates the productivity of water • Also called as “productivity index” Calculation : Cm = D 1+D 2 2

• • The calculated depth is also called as Secchi disc transparency It indicates the appropriate idea about the concentration of suspended matter in water using table value Transparency Suspended materials (ppm ) 2. 0 1000 2. 6 700 3. 0 500 4. 0 360 5. 0 240 6. 0 190 7. 0 110 9. 00 10. 00 11. 00 90 12. 00 80 15. 00 65 18. 00 50 20. 00 45 22. 00 40 25. 00 35 • Turbidity of natural water seldom exceeded 20, 000 ppm • Desirable range of turbidity for aquaculture is between 20 -40 cm

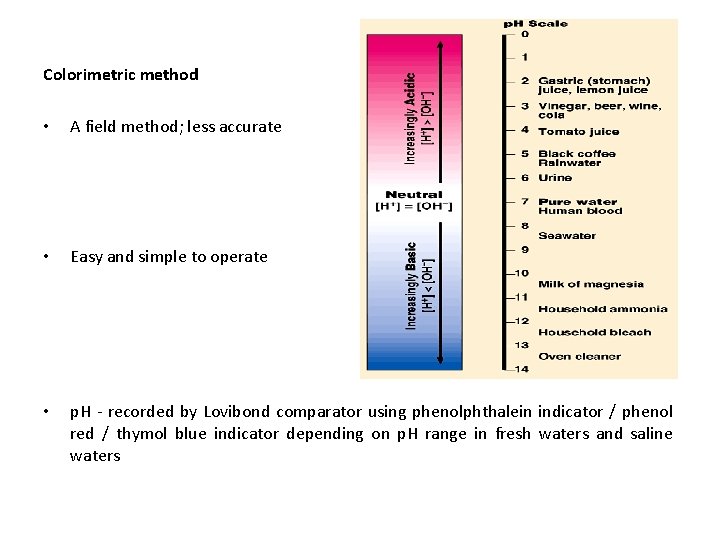
p. H • p. H of water - determines the pond productivity • p. H affects the physiological functions of fishes in water • Also affects the equilibrium of hydroxide, carbonate, phosphate and silicate between bottom soil and overlying water • The desirable p. H for fish pond production is between 7. 5 and 8. 5 • p. H - measures the concentration of hydrogen ion in the water • p. H - measured colorimetrically and potentiometrically

Colorimetric method • A field method; less accurate • Easy and simple to operate • p. H - recorded by Lovibond comparator using phenolphthalein indicator / phenol red / thymol blue indicator depending on p. H range in fresh waters and saline waters

Potentiometric method • The method is more accurate; requires less time; widely used • p. H is recorded by immersing the standardized p. H meter by following guidelines of the manufacture Note: p. H meter is calibrated with p. H values of 4. 0, 7. 0 and 9. 2 standard p. H solutions prepared by using the tablets of each p. H

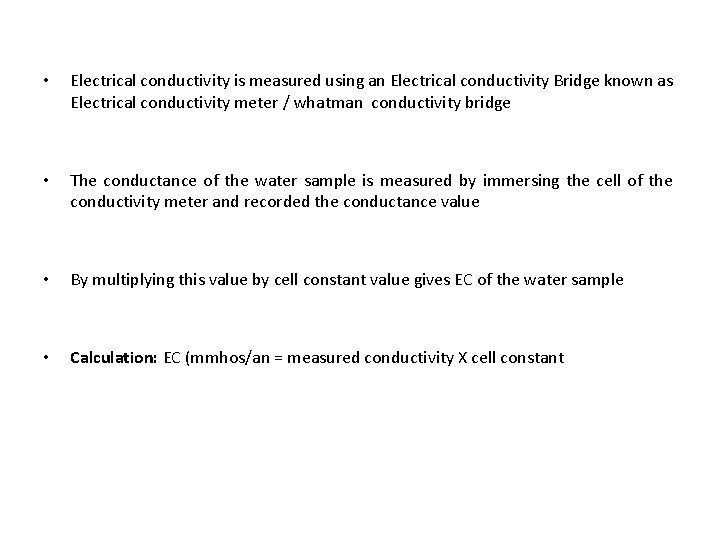
ELECTRICAL CONDUCTIVITY • EC-indicates the total concentration of ionized constituents in water • Closely related to the amount of total dissolved solids • EC - used as an index of salt content of water • EC is reciprocal of electrical resistance • In natural water EC values normally ranges from 20 to 1500 mmhos /cm • C = I/2 R Where as R is resistance

• Electrical conductivity is measured using an Electrical conductivity Bridge known as Electrical conductivity meter / whatman conductivity bridge • The conductance of the water sample is measured by immersing the cell of the conductivity meter and recorded the conductance value • By multiplying this value by cell constant value gives EC of the water sample • Calculation: EC (mmhos/an = measured conductivity X cell constant

SALINITY • Salinity of freshwater - very low i. e. < 0. 5 • Salinity of water is the total concentration of soluble salts in a litre of water and expressed as ppt (part of per thousand) • Mohr’s method is used the measuring the chlorinity of water • Salinity of water can be calculated from chlorinity or electrical conductivity values of water • Salinity (ppt) = Chlorinity X 1. 805 + 0. 03

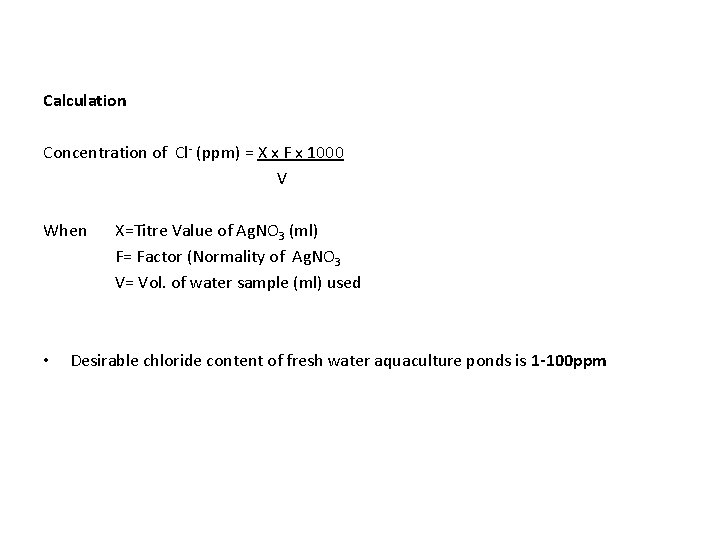
CHLORIDE • The chloride (Cl-) in water indicates the total amount of soluble salts • Chloride ions can be estimated by titration with Silver Nitrate (Ag. NO 3) in the presence of chloride ions Silver Chloride forms Silver chloride (Ag. Cl) by reacting with the Chromate (Cr. O 42 -) present in water • When the chloride in water becomes exhausted, Ag. NO 3 then reacts with the Cr. O 42 - ions to form a red color of Silver chromate (Ag. Cro 4) indicating the reaction has been completed

Calculation Concentration of Cl- (ppm) = X x F x 1000 V When • X=Titre Value of Ag. NO 3 (ml) F= Factor (Normality of Ag. NO 3 V= Vol. of water sample (ml) used Desirable chloride content of fresh water aquaculture ponds is 1 -100 ppm

TOTAL SOLIDS • Total solids in the water sample represent the dissolved organic matter, particulate organic matter, and dissolved inorganic substances • Different forms of solids present in water are: – Total solids (TS); – Total dissolved solids (TD); – Total suspended solids (TSS); – Total volatile solids (TVS) or Total volatile dissolved solids (TVDS).

• For determination of total solids, heat a clean procelin basin in an oven at 1050 c, cool and weigh – W 1. • Transfer well mixed 100 ml. of sample in the basin • Evaporate the sample to dryness in oven at 1000 c. • Cool it in desiccators and then weigh the residue – W 2

• For TDS, filter the water sample (100 ml) with filter paper and then filtrate on the filter is dried – W 3 • For TVS, ignite the residue (W 2) the total solids in the muffle furnace at 5500 C for half an hour • Cool in a desiccator and weigh – W 4 • For TVDS, ignite the residue of TDS as above, cool in a desssicator and weigh – W 5

Calculation: TS (ppm) = W 2 – W 1 mg x 10 TDS (ppm) = W 3 –W 1 mg x 10 TVS (ppm) = W 2 –W 4 mg x 10 TVDS (ppm) = W 3 – W 5 mg x 10

TOTAL HARDNESS • Hardness of water is caused principally due to the elements of Calcium and Magnesium and sometimes by Iron and Aluminum • Determination of Ca++ and Mg++ usually provides a fair idea about hardness of water and is expressed in terms of calcium carbonate (Ca. CO 3)

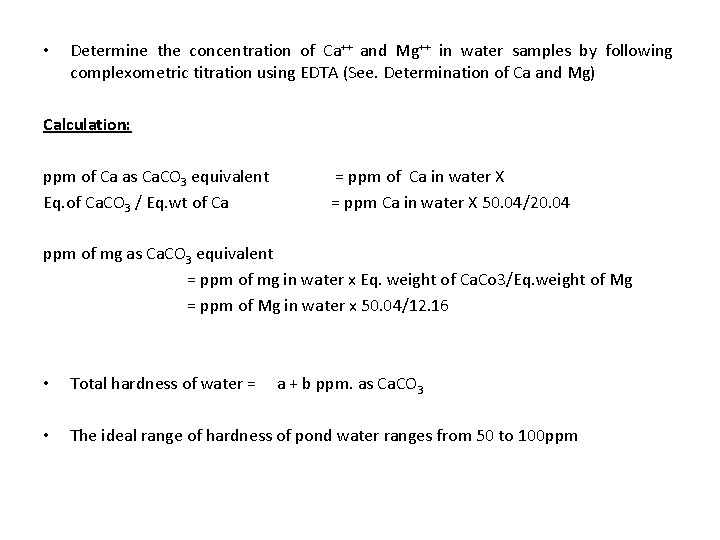
• Determine the concentration of Ca++ and Mg++ in water samples by following complexometric titration using EDTA (See. Determination of Ca and Mg) Calculation: ppm of Ca as Ca. CO 3 equivalent = ppm of Ca in water X Eq. of Ca. CO 3 / Eq. wt of Ca = ppm Ca in water X 50. 04/20. 04 ppm of mg as Ca. CO 3 equivalent = ppm of mg in water x Eq. weight of Ca. Co 3/Eq. weight of Mg = ppm of Mg in water x 50. 04/12. 16 • Total hardness of water = a + b ppm. as Ca. CO 3 • The ideal range of hardness of pond water ranges from 50 to 100 ppm

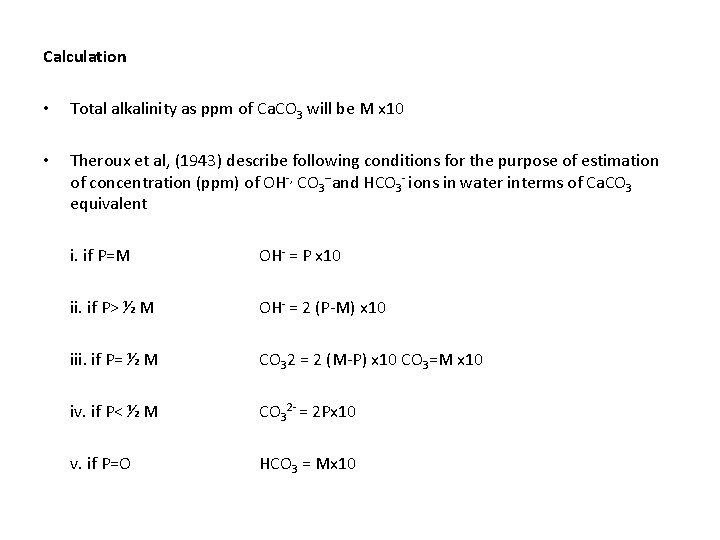
TOTAL ALKALINITY • Alkalinity - the amount of acid required to neutralize the bases in water(Boyd, 1978) • Hydroxides (OH-), carbonates (CO 3 2 - ) bicarbonates (HCO 3 -), ammonia (NH 3+), silicate (Sio 3) and phosphate (PO 4 -) etc. - contribute to the alkalinity of water • Bases which contribute considerably to alkalinity are anions such as< OH- , CO 32 - and HCO-3 • Alkalinity of water is determined by titrating water sample with 0. 02 N H 2 SO 4 using phenolphthline and methyl orange indicators • Note down the end point of phenolphthaline titre value( P) and methyl organge titre value(M)TY

Calculation • Total alkalinity as ppm of Ca. CO 3 will be M x 10 • Theroux et al, (1943) describe following conditions for the purpose of estimation of concentration (ppm) of OH-, CO 3 --and HCO 3 - ions in water interms of Ca. CO 3 equivalent i. if P=M OH- = P x 10 ii. if P> ½ M OH- = 2 (P-M) x 10 iii. if P= ½ M CO 32 = 2 (M-P) x 10 CO 3=M x 10 iv. if P< ½ M CO 32 - = 2 Px 10 v. if P=O HCO 3 = Mx 10

DISSOLVED OXYGEN • Dissolved oxygen (DO 2) is an important chemical parameter • DO is used by aquatic organisms for respiration • DO - govern the productivity of the aquatic ecosystem • Estimated by methods such as Winkler’s method (Winkler’s, 1888) and polarographic technique • Winkler’s method is widely used

• Manganous hydroxide (Mn. OH 2) reacts with DO of water under highly alkaline condition (Alkaline iodide) • An equivalent amount of manganeous (Mn 2+) is oxidized to higher valancy state • On acidification, in the presence of iodide, the oxidized manganous ( Mn 4+) again reverts to divalent Mn 2+ and iodide (I 2) is liberated in equivalent amount of DO • This I 2 estimated by titration with Sodium thiosulphate (Na 2 S 2 O 3) with starch indicator indicates the amount of DO in the water sample Calculation • DO in water sample (mg/l) = ml of 0. 025 N. Na 2 S 2 O 3 x 4 • Desirable DO level should be 5. 0 ppm or more for fish production in ponds

Polarographic method • This method of estimation is rapid and direct polarographic oxygen analysis • Consists of probe comprising of two electrodes immersed in potassium chloride and separated from the water sample by a membrane • The current produced by electrode is proportional to the tension of oxygen in water

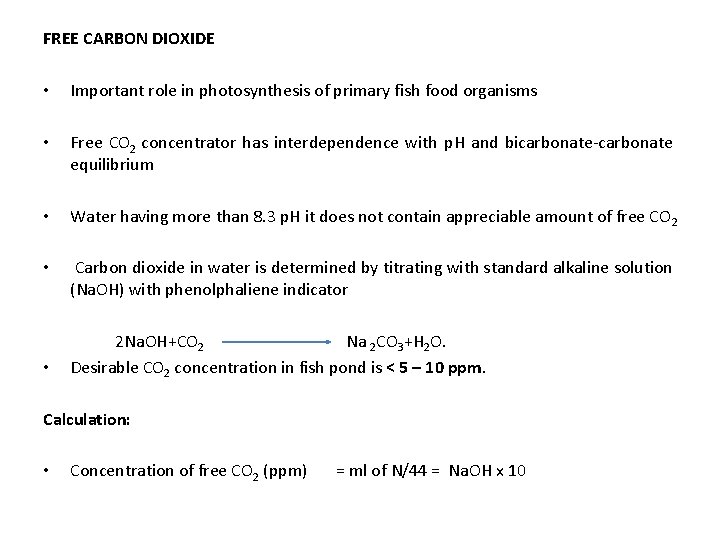
FREE CARBON DIOXIDE • Important role in photosynthesis of primary fish food organisms • Free CO 2 concentrator has interdependence with p. H and bicarbonate-carbonate equilibrium • Water having more than 8. 3 p. H it does not contain appreciable amount of free CO 2 • Carbon dioxide in water is determined by titrating with standard alkaline solution (Na. OH) with phenolphaliene indicator 2 Na. OH+CO 2 Na 2 CO 3+H 2 O. • Desirable CO 2 concentration in fish pond is < 5 – 10 ppm. Calculation: • Concentration of free CO 2 (ppm) = ml of N/44 = Na. OH x 10

AMMONIUM- NITROGEN AND NITRATE-N • • Inorganic forms of N dissolved in water are present predominantly in Ammonium (NH 4+) and Nitrate (N 2 -) both of these NH 4+ and NO 3 -) - readily available to primary fish food organisms Ammonium and Nitrate of N can be determined by using colorimetric methods using Nesslers reagent and phenol disulfonic acid respectively • Inorganic nitrogen (NH 4+, NO 3 -) below 0. 1 ppm - as poor productivity of pond • Inorganic nitrogen value 0. 2 ppm - good productivity (Banerge, 1967)

PHOSPHATE • Phosphate (P) - most critical nutrient element for good pond productivity (Jhingran 1975) • P -essential nutrient for primary fish food organisms • Phosphorus in water is estimated colorimetrically after development of phosphomolybolic blue color • Ascorbic acid and stannous chloride have been as the reducing agent for developing color • Measure OD at 660 nm wavelength using spectrophotometer • Optimum concentration in fish pond water: > 2. 0 ppm, 0. 05 - 2. 0 ppm -moderate and < 0. 05 ppm- poor
