STERILIZATION HEALTHCARE Steam Sterilizers Operation and Preventative Maintenance





















- Slides: 29

STERILIZATION HEALTHCARE Steam Sterilizers Operation and Preventative Maintenance

Sterilization Fundamentals Sterilization: A process by which all forms of microbial life including bacteria, viruses, spores, and fungi are destroyed v. Note: In a sterilization process, the nature of microbial death is described by an exponential function. Therefore, the presence of microorganisms on any individual item can be expressed in terms of probability. While this probability can be reduced to a very low number, it can never be reduced to zero. (ANSI/AAMI ST 46)

General Terminology Review Sterility Testing: Product is inoculated directly into growth medium. Process Monitoring: Use of mechanical, biological and chemical indicators to monitor critical sterilization parameters. Parametric release: Declaring product sterile based on records demonstrating that the process parameters were delivered within specified tolerances. This method does not include the use of Biological Indicators.

General Terminology Review • Bio Burden – # of microorganisms on a contaminated item • Reduction of Bio Burden: – – – Hand washing; soap and water Chemical; high level disinfection Sanitization Decontamination Disinfection

What is a Steam Sterilizer ?

Technology Fundamentals Steam sterilizers for healthcare have one primary purpose: Render instruments sterile for re-use in a surgical setting

Sterilization Fundamentals Steam Sterilizers – Temperatures ranging from 121 -134 C at pressures of 15 -30 psi are generally recommended to sterilize wrapped or unwrapped surgical instruments. – Why Steam? Easy to Control Inexpensive Non Toxic Highly Effective Readily Available

Sterilization Fundamentals Two Types of Steam Sterilizers: Vacuum: used for large volumes of wrapped surgical instruments Gravity: used for individual unwrapped surgical instruments

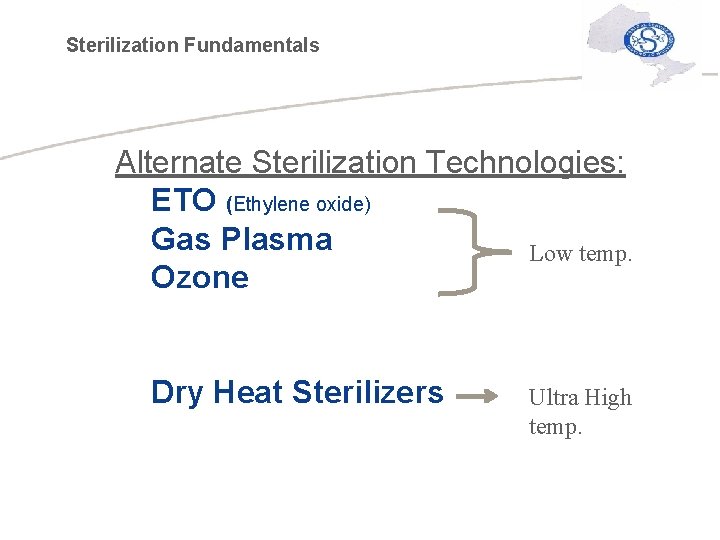
Sterilization Fundamentals Alternate Sterilization Technologies: ETO (Ethylene oxide) Gas Plasma Low temp. Ozone Dry Heat Sterilizers Ultra High temp.

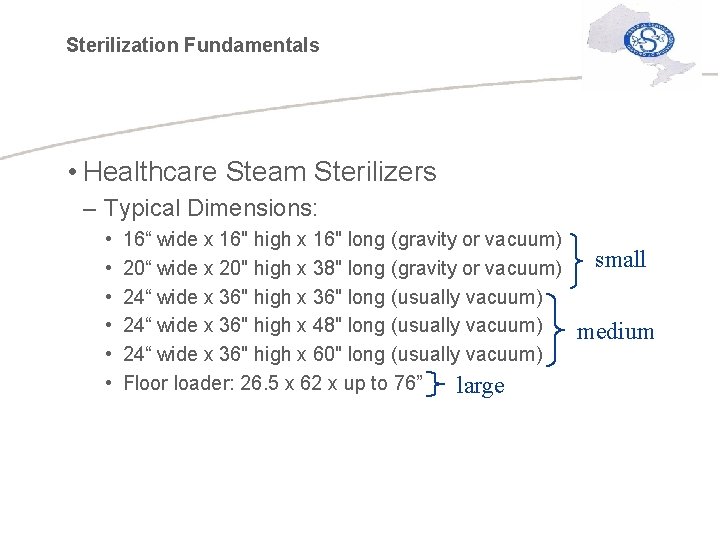
Sterilization Fundamentals • Healthcare Steam Sterilizers – Typical Dimensions: • • • 16“ wide x 16" high x 16" long (gravity or vacuum) 20“ wide x 20" high x 38" long (gravity or vacuum) 24“ wide x 36" high x 36" long (usually vacuum) 24“ wide x 36" high x 48" long (usually vacuum) 24“ wide x 36" high x 60" long (usually vacuum) Floor loader: 26. 5 x 62 x up to 76” large small medium

Sterilization Fundamentals • Steam Sterilizers – Smaller sterilizers: instruments inserted individually for quick sterilization – Larger sterilizers (36" or larger): can hold entire “carriage” of instruments for sterilizing – Floor loader: rises to floor level where entire cart and carriage of instruments are inserted for sterilization

Sterilization Fundamentals Factors Affecting Sterilization • Time – Sterility Assurance Level (SAL) 10 -6 – One survivor out of a million (infectious organisms) • Temperature – 250 F/275 F • Moisture – Required to coagulate protein – However, steam quality requires a maximum vapor content of 3%

Sterilization Fundamentals Steam Quality Through RO Water • Reverse-Osmosis (RO): the process of squeezing water through a semi-permeable membrane. • RO will separate pure water molecules from dissolved solids such as Calcium, Sodium and Iron • Chlorine, Nitrates and Nitrites are removed in the pre-filtration process.

Sterilization Fundamentals Benefits of RO in Steam Generation ( Clean Steam) üBalances p. H üReduces downtime üReduces costly repairs üExtends useful equipment life üRemoves hardness üImproves equipment reliability

Sterilization Fundamentals Other Factors Affecting Sterilization üProper sterilizer design and use to achieve time and temperature üSterilizer area design and quality utilities feeding unit üBio Burden reduced prior to sterilization üAdequate contact of the sterilant for all surfaces

Sterilization Fundamentals Why is air removal important? ØAir and steam do not mix ØAir inhibits steam contact ØAir is 1. 6 times denser than steam ØSteam condenses on cool goods ØLoading and load size ØPacks must be dry

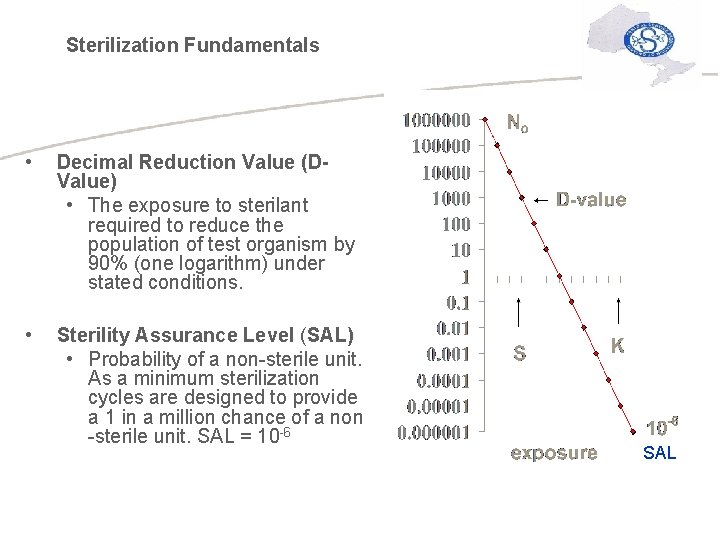
Sterilization Fundamentals • Decimal Reduction Value (DValue) • The exposure to sterilant required to reduce the population of test organism by 90% (one logarithm) under stated conditions. • Sterility Assurance Level (SAL) • Probability of a non-sterile unit. As a minimum sterilization cycles are designed to provide a 1 in a million chance of a non -sterile unit. SAL = 10 -6 SAL

Sterilization Fundamentals More about D-Value and SAL. . . üThe SAL is extrapolated from the survivor curve based on the D-value of the Biological Indicator and exposure time. üTraditional cycle development is driven by determining the time for complete kill of a 106 BI and double the cycle time to achieve SAL of 10 -6

Sterilization Fundamentals 3 Primary Factors of Sterility Assurance 1. Process Control - Reproducible and controlled conditions 2. Process Monitoring - Biological indicators, chemical indicators, mechanical gauges, cycle charts or printouts, etc. 3. Good Manufacturing Practices (GMP) - Includes record keeping, inspection of materials, process validation and equipment maintenance and calibration Complete sterility assurance can only be achieved by testing each item processed - a destructive and impractical procedure.

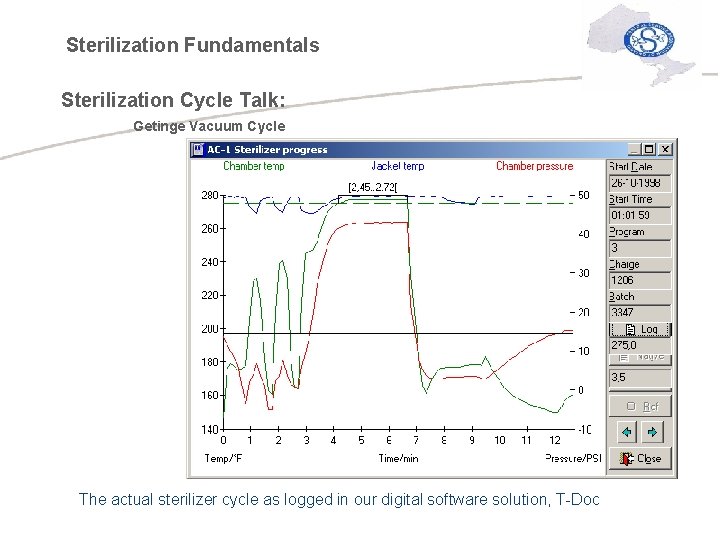
Sterilization Fundamentals Sterilization Cycle Talk: Getinge Vacuum Cycle The actual sterilizer cycle as logged in our digital software solution, T-Doc

Sterilizer Maintenance • Why should we have preventative maintenance on a sterilizer? • What happens in the sterilizer during a cycle? • What should I look for the start of my shift? • What can effect the sterilization process that I can control? • What can effect the sterilization process that I can not control directly?

Sterilizer Maintenance Why should we have preventative maintenance on a sterilizer? 1. Keeps the machine running to manufactures recommended specifications 2. Minimizes the amount of downtime 3. Provides assurance that the unit is running optimally 4. Extends the life of the sterilizer 5. Ensures the unit is safe for operating- no chamber cracks, faulty valves etc 6. Ensures the appropriate sterility is met by the system as it was intended to provide at time of manufacturing

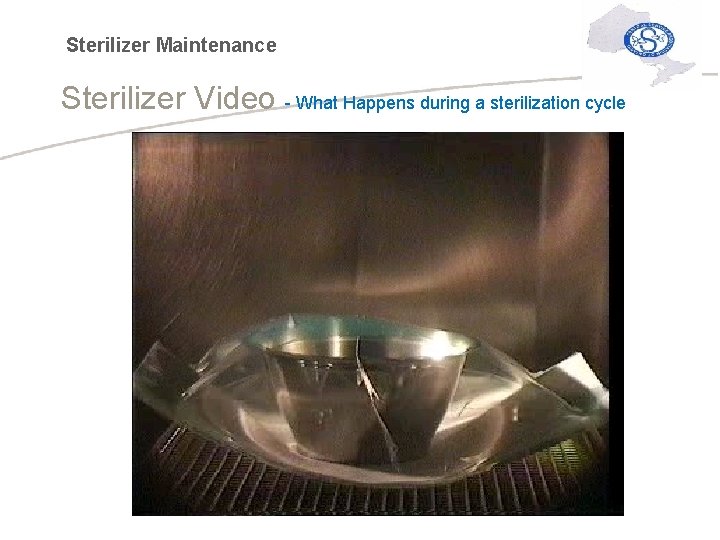
Sterilizer Maintenance Sterilizer Video - What Happens during a sterilization cycle

Sterilizer Maintenance What should I look for when start of my shift? 1. 2. 3. 4. 5. 6. Ask the person who you are replacing if there have been any autoclave problems during their shift. Keep a log book of events for of date, time, unit number, copy of print out and what occurred. Check gasket for wear and tear on the machines you are using Check chamber and drains to ensure no paper or items are obstructing the drain Check the printer paper to ensure you have enough Verify you have appropriate materials for logging, biological tests and bowie dick tests if required for your shift Check to see if you’ll be required to process stat loads etc during your work period to allow you to manage the flow

Sterilizer Maintenance What can effect the sterilization process that I can control? 1. The proper loading and spacing of the sterilizer cart/trolley 2. Using the proper packaging material 3. Checking to ensure drain is not plugged and is cleaned at regular intervals 4. Cleaning of the chamber and carts at regular intervals 5. Proper cool down area away from air conditioning 6. Items being sterilized are dry, containers, blue silicone liners dry as well as plastic bowls etc 7. Being able to properly read the tests – BD, Biological, Indicators, Integrators etc 8. Prequalification of the units after a major repair of the sterilizer

Sterilizer Maintenance What can effect the sterilization process that I can not control directly? 1. 2. 3. 4. 5. 6. 7. The sterilizer malfunctioning Steam supply staturation drops below 97% Water pressure drops Electrical power failure Failure of BD Test – redo test and check results – 2 nd failure contact service for repair Consistently wet steam or dirty steam – (tea effect) – You can perform a warm-up prior to the bowie dick test and this will help remove as much moister from the lines as possible. Dirty steam – you can have filters placed at the feed of the units to filter out debris and some of the chemicals used by maintenance to clean the steam lines

Sterilization Fundamentals Who establishes the proper sterilization parameters for reusable medical devices? Answer: The responsibility for safe and effective sterilization of reusable medical devices is the shared responsibility of both manufacturers, and users of the devices. Reichert & Young, p. 132

Summary - Key Learnings • Reviewed General Terminology • Reviewed the Sterilization Fundamentals • Reviewed Sterilizer Maintenance and areas that can be controlled by MDRD employee

Sterilizer Maintenance Questions? Thank you for your Time Documents%20 and%20 Settings/Rdemedeiros/My%2 0 Documents/cyberclean. html
 Maintenance of milling machine
Maintenance of milling machine Heat recovery steam generator fouling
Heat recovery steam generator fouling Dittus-boelter equation
Dittus-boelter equation Steam steam generator erosion
Steam steam generator erosion Corrective facework
Corrective facework Don bosco preventive system
Don bosco preventive system Healthcare and the healthcare team chapter 2
Healthcare and the healthcare team chapter 2 Sports medicine definition
Sports medicine definition Operation and maintenance dairy plant
Operation and maintenance dairy plant Boiler operation and maintenance training ppt
Boiler operation and maintenance training ppt Mbbr operation and maintenance manual
Mbbr operation and maintenance manual Differentiate between disinfection and sterilization
Differentiate between disinfection and sterilization Sterilization and disinfection
Sterilization and disinfection Sterilization and disinfection
Sterilization and disinfection Sterilization and disinfection
Sterilization and disinfection Sterilization
Sterilization Nurses responsibility in sterilization
Nurses responsibility in sterilization Batch and continuous sterilization
Batch and continuous sterilization Sterilization and disinfection
Sterilization and disinfection Low level disinfectant
Low level disinfectant Tindalization
Tindalization Sterilization and disinfection in microbiology lab
Sterilization and disinfection in microbiology lab Principles of hot air oven
Principles of hot air oven Sterilization by mechanical methods
Sterilization by mechanical methods Principle of autoclave
Principle of autoclave What is sterilization
What is sterilization Principle of autoclave ppt
Principle of autoclave ppt Instrument processing
Instrument processing What is sterelization
What is sterelization Physical sterilization
Physical sterilization