STANDARD OPERATING PROCEDURE 1 INTRODUCTION A Standard Operating




















- Slides: 27

STANDARD OPERATING PROCEDURE 1

INTRODUCTION A Standard Operating Procedure (SOP) is a set of written instructions that document a routine or repetitive activity followed by an organization. ICH defines SOP as “detailed written instructions to achieve uniformity of the performance of a specific function”. Other terms may also be used instead of the term SOP Protocols Instructions Worksheets Safe operating procedures ü ü 2

PURPOSE The basic purpose of SOP is to detail the regularly recurring work processes , that are to be conducted of followed within an organisation. To achieve uniformity of the performance of a specific function. Enable the people to perform their functions accurately and without mistakes. SOPs are tools to ensure that GMP are followed wherever applicable. To ensure highest standards for drug products, that have to be ultimately consumed by the patient. 3

BENEFITS Promotes quality through consistent implementation of a process or procedure within the organization. It facilitates consistency in the quality and integrity of a product or end-result. It provides information about how to perform a job properly and minimizes variations. Address safety concerns Ensures that processes continue uninterrupted 4 It is used as a part of a personnel training program.

OBLIGATIONS WHO has already made it mandatory to have the required SOPs for any manufacturing facility for grant of GMP certificate. Various legal and commercial factors also influence the manufacturer to go in providing SOPs for his facilities. 5

WRITING STYLES SOPs should be written in a concise, step-by-step, easyto-read format. The information presented should not be overly complicated. It should be simple and short and should be conveyed clearly. The active voice and present tense verb should be used Use a flow chart to illustrate the process being described (since it is very easy to understand). 6

7

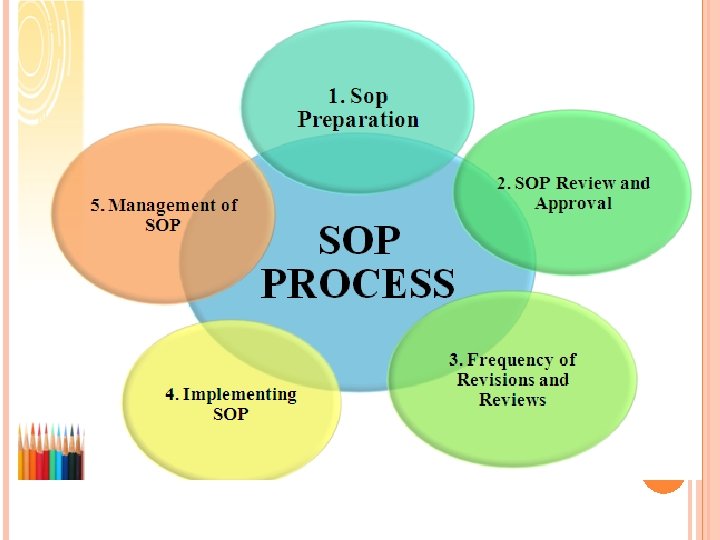
SOP Preparation q The organization should have a procedure in place for determining what procedures or processes need to be documented. q SOPs should be written by an individual who performs the tasks routinely or someone who is directly responsible for the performance of the task. 8

SOP Review and Approval ØSOPs should be reviewed by one or more individuals with appropriate training and experience with the process especially helpful if draft SOPs are actually tested by individuals other than the original writer before the SOPs are finalized. 9

Frequency of Revisions and Reviews ØSOPs need to remain current to be useful. Therefore, whenever procedures are changed, SOPs should be updated and re-approved. If desired, modify only the pertinent section of an SOP and indicate the change date/revision number for that section in the Table of Contents and the document control notation. ØSOPs should be also systematically reviewed on a periodic basis, e. g. every 1 -2 years, to ensure that the policies and procedures remain current and appropriate, or to determine whether the SOPs are even needed. 10

Implementing SOP q The most important step for implementing the SOP is in working area, train or retrain the user. Everyone should follow the procedure exactly with each and every step in detail, it is very important to train the user otherwise individual may interpret meaning in different ways. q While training the user trainer should share the reason WHY SOP must performed correctly. People are much more to follow when they understand importance of procedure. q Trainer should explain and demonstrate how each step in the SOP will be performed and should assure them this will increase Quality of product by providing safety and accuracy which will ultimately 11 increase the confidence of the user.

Management of SOP Ø Organization shall have SOP on Preparation, approval, revision and control of standard Operating Procedure for better control and management of SOPs. Ø Generally, administrative aspects of the SOP system such as distribution and filing are well managed. On the other hand, overall system management, frequently characterized by the lack of a system owner, is generally poor. If a system owner exists at all, his or her responsibilities are limited. 12

Continued…. Ideally a system owner ØEliminates obsolete SOPs. (Which is not needed) ØEnsures that SOPs meet their quality requirements and are user friendly. ØManages SOP change controls. ØDistributes SOPs. ØEnsures that SOPs are current. ØEnsures that new or changed SOPs are valid only after training has occurred and provides training about the SOP system. ØMeasures system performance and periodically reports results to management. ØContinuously improves the system. 13

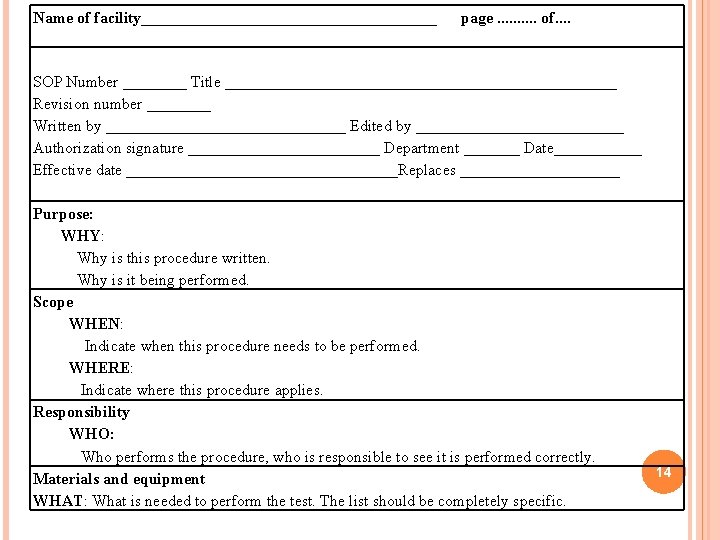
Name of facility___________________ page. . of. . SOP Number ____ Title _________________________ Revision number ____ Written by _______________ Edited by _____________ Authorization signature ____________ Department _______ Date______ Effective date _________________Replaces __________ Purpose: WHY: Why is this procedure written. Why is it being performed. Scope WHEN: Indicate when this procedure needs to be performed. WHERE: Indicate where this procedure applies. Responsibility WHO: Who performs the procedure, who is responsible to see it is performed correctly. Materials and equipment WHAT: What is needed to perform the test. The list should be completely specific. 14

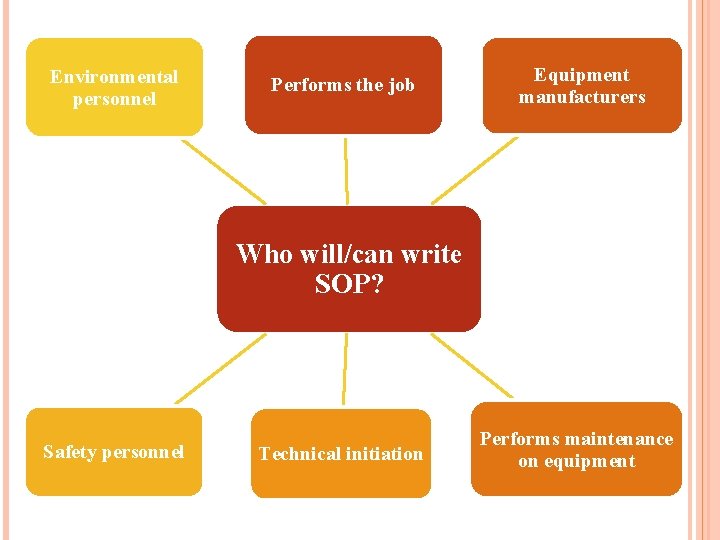
Environmental personnel Performs the job Equipment manufacturers Who will/can write SOP? Safety personnel Technical initiation Performs maintenance on equipment 15

SOP GENERAL FORMAT SOPs should be organized to ensure ease and efficiency in use and to be specific to the organization which develops it. Where possible break the information into a series of logical steps to avoid a long list. SOP generally consists of 1. Title Page 2. Table of Contents 3. Text 16

1. TITLE PAGE The first page or cover page of each SOP should contain the following information: a. A title that clearly identifies the activity or procedure b. An SOP identification (ID) number c. Date of issue and/or revision d. The signatures and signature dates of those individuals who prepared and approved the SOP. 17

2. TABLE OF CONTENTS A Table of Contents may be needed for quick reference, especially if the SOP is long • T o locate the information • To denote changes or revisions made only to certain sections of an SOP. 18

3. TEXT Well-written SOPs should first briefly describe a. The purpose of the work or process b. The scope of the work or process c. Responsibilities and applicabilities d. Summary of the method/procedure e. Definition of any specialized/unusual terms and explanation of abbreviations. 19

f. Health and safety cautions g. QC Section: Used to check the Quality of the work that include specific assessment criteria and appropriate QC procedures. f. Attach any appropriate information, e. g. , an SOP may reference other SOPs. 20

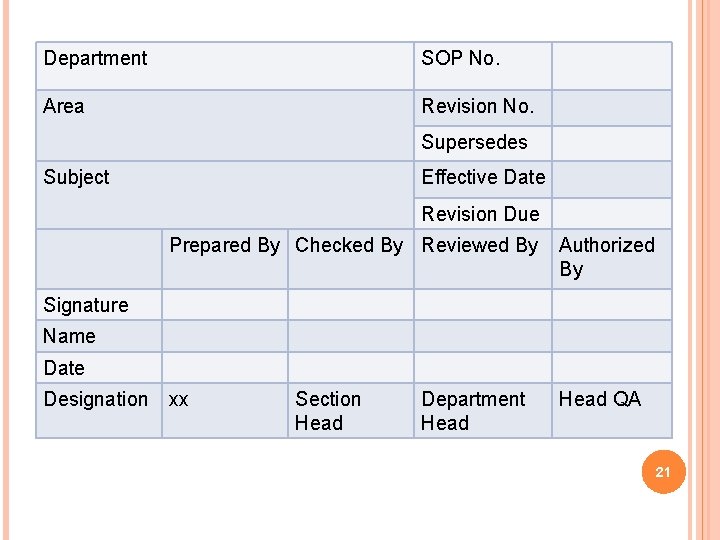
Department SOP No. Area Revision No. Supersedes Subject Effective Date Revision Due Prepared By Checked By Reviewed By Authorized By xx Head QA Signature Name Date Designation Section Head Department Head 21

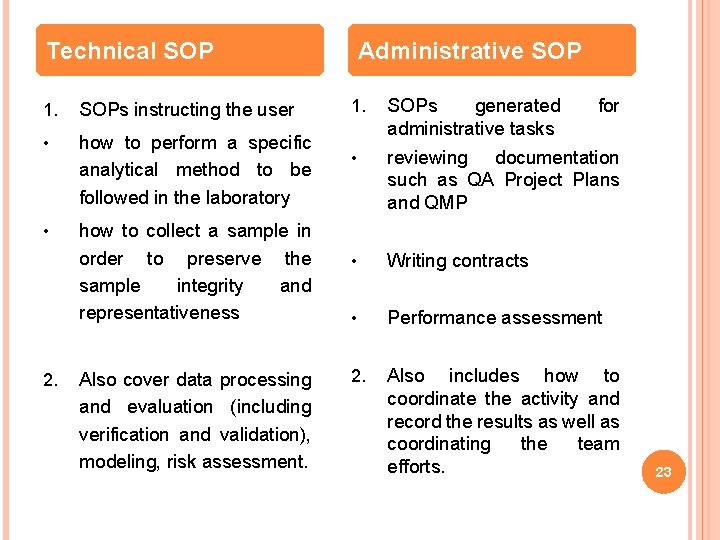
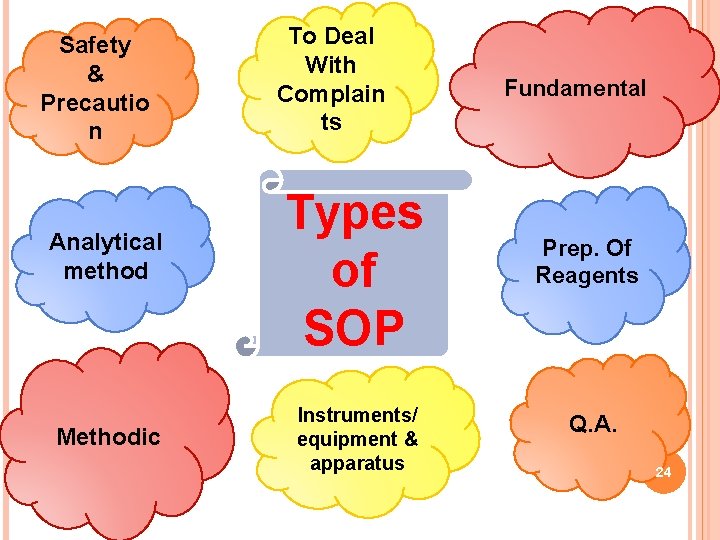
TYPES OF SOPS SOPs may be written for any repetitive technical activity, as well as for any administrative or functional programmatic procedure, that is being followed within an organization. The types of SOPs are: 1. Technical SOP 2. Administrative /Fundamental Programmatic SOP 22

Technical SOP 1. SOPs instructing the user • how to perform a specific analytical method to be followed in the laboratory • how to collect a sample in order to preserve the sample integrity and representativeness 2. Also cover data processing and evaluation (including verification and validation), modeling, risk assessment. Administrative SOP 1. SOPs generated administrative tasks for • reviewing documentation such as QA Project Plans and QMP • Writing contracts • Performance assessment 2. Also includes how to coordinate the activity and record the results as well as coordinating the team efforts. 23

Safety & Precautio n Analytical method Methodic To Deal With Complain ts Types of SOP Instruments/ equipment & apparatus Fundamental Prep. Of Reagents Q. A. 24

MASTER SOP In addition to the various SOPs that are required, the company has to first make an SOP that defines how the various SOPs will be made, i. e. what kind of information, structure and numbering system will be included in various SOPs. It should also contain a time frame for revision of SOPs. It should identify the persons authorized for each activity (creating, checking, verifying and implementing). 25

REFERENCES 1. 2. 3. 4. 5. 6. 7. 8. http: //www. pharmainfo. net/reviews/standard operatingprocedures http: //www. epa. gov/QUALITY/qs-docs/g 6 -final http: //en. wikipedia. org/wiki/Standard_operating_proced ure http: //www. sop-standard-operating-procedure. com http: //www. meathaccp. wisc. edu. http: //www. rfnozick. com/services. cfm? t=15 http: //www. ezinearticles. com>home>business Good Laboratory Practice, Alex D. Kanarek, 3 rd Edition, M and MD Publications. 26

27