Specific Heat The amount of heat energy needed









- Slides: 15

Specific Heat The amount of heat energy needed to raise the temperature of a 1. 0 g of a substance 1 o. C.

• Substances with low specific heats absorb & release heat E quickly –Ex. Metals Aluminum 0. 9 J/go. C • Those with high specific heats absorb & release heat E slowly –Ex. Water 4. 18 J/go. C

• Variables & their units. . . – q is Heat energy absorbed or released measured in Joules – Specific Heat (C) units: J/go. C – Heat of fusion (Hf ) E needed to melt one gram of a substance at constant temperature. – Heat of vaporization (Hv) E needed to vaporize one gram of a substance at constant temperature.

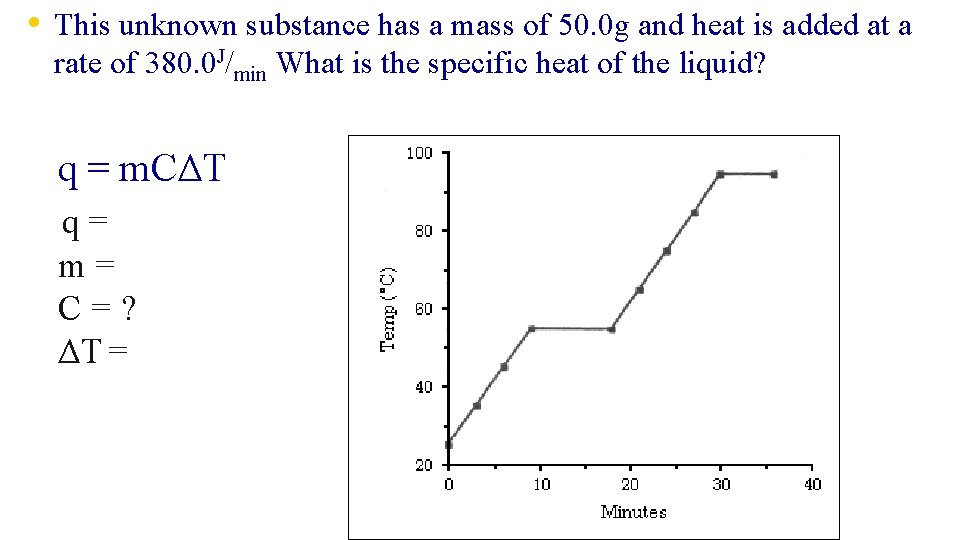
• This unknown substance has a mass of 50. 0 g and heat is added at a rate of 380. 0 J/min What is the specific heat of the liquid? q = m. CΔT q= m= C=? ΔT =

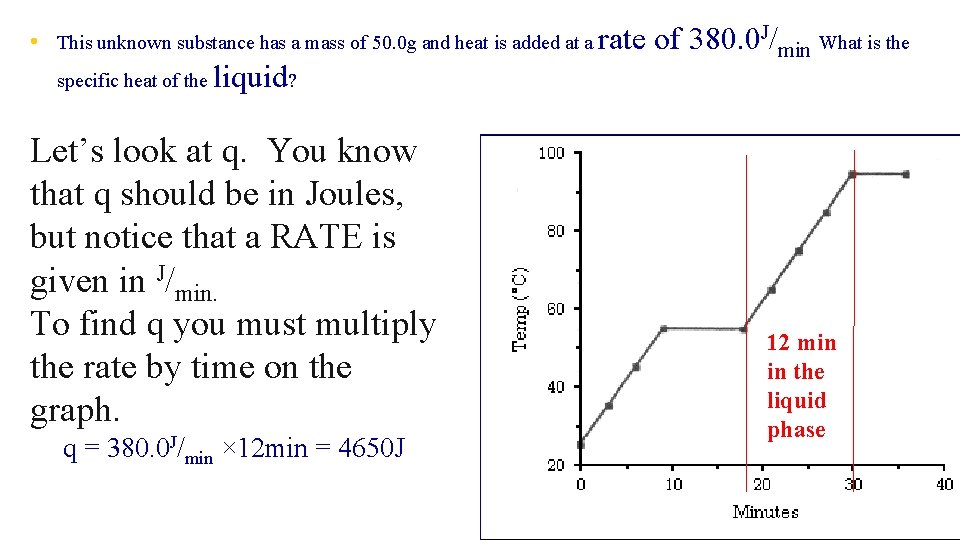
• This unknown substance has a mass of 50. 0 g and heat is added at a rate specific heat of the liquid? Let’s look at q. You know that q should be in Joules, but notice that a RATE is given in J/min. To find q you must multiply the rate by time on the graph. q = 380. 0 J/min × 12 min = 4650 J of 380. 0 J/min What is the 12 min in the liquid phase

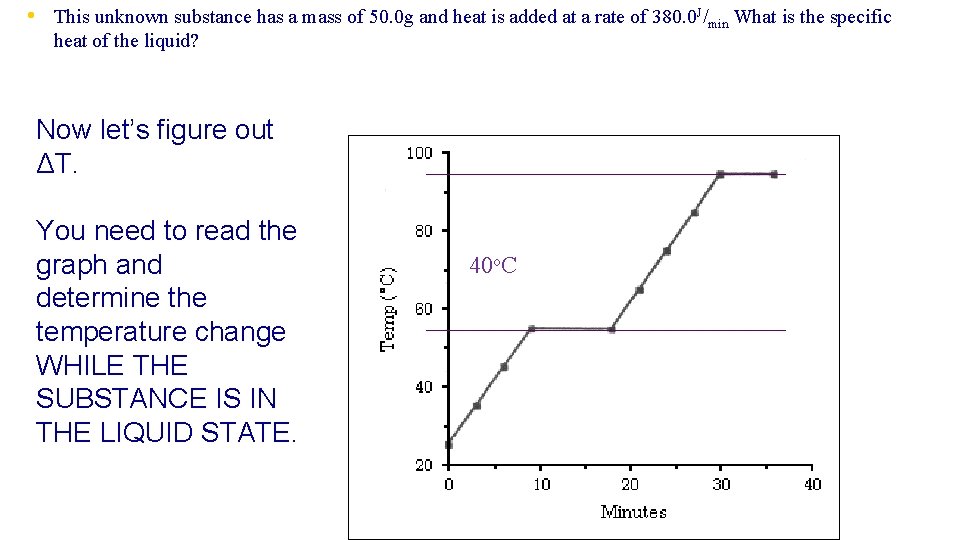
• This unknown substance has a mass of 50. 0 g and heat is added at a rate of 380. 0 J/min What is the specific heat of the liquid? Now let’s figure out ΔT. You need to read the graph and determine the temperature change WHILE THE SUBSTANCE IS IN THE LIQUID STATE. 40 o. C

• This unknown substance has a mass of 50. 0 g and heat is added at a rate of 380. 0 J/min What is the specific heat of the liquid? The mass is given in the problem, so now you can substitute and solve. q = m. CΔT q = 4560 J m = 50 g C=? ΔT = 40 o. C o 2. 28 J/g C

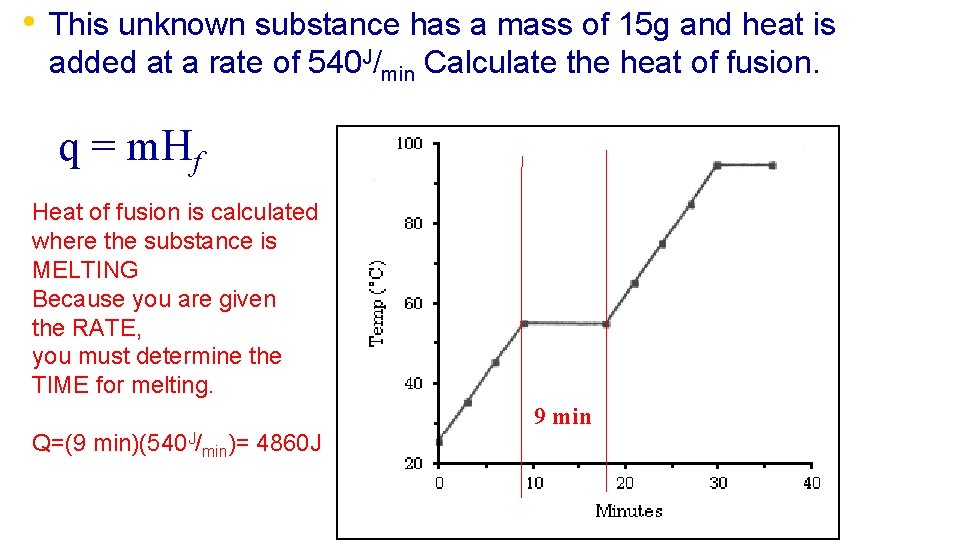
• This unknown substance has a mass of 15 g and heat is added at a rate of 540 J/min Calculate the heat of fusion. q = m. Hf Heat of fusion is calculated where the substance is MELTING Because you are given the RATE, you must determine the TIME for melting. Q=(9 min)(540 J/min)= 4860 J 9 min

• This unknown substance has a mass of 15 g and heat is added at a rate of 540 J/min Calculate the heat of fusion. Now substitute and solve. q = m. Hf q = 4860 J m = 15 g Hf =? 324 J/g

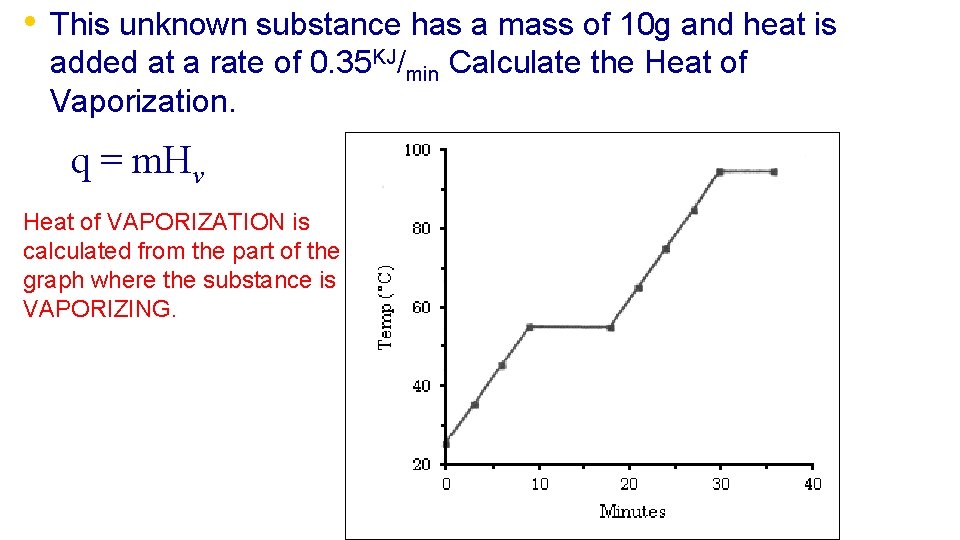
• This unknown substance has a mass of 10 g and heat is added at a rate of 0. 35 KJ/min Calculate the Heat of Vaporization. q = m. Hv Heat of VAPORIZATION is calculated from the part of the graph where the substance is VAPORIZING.

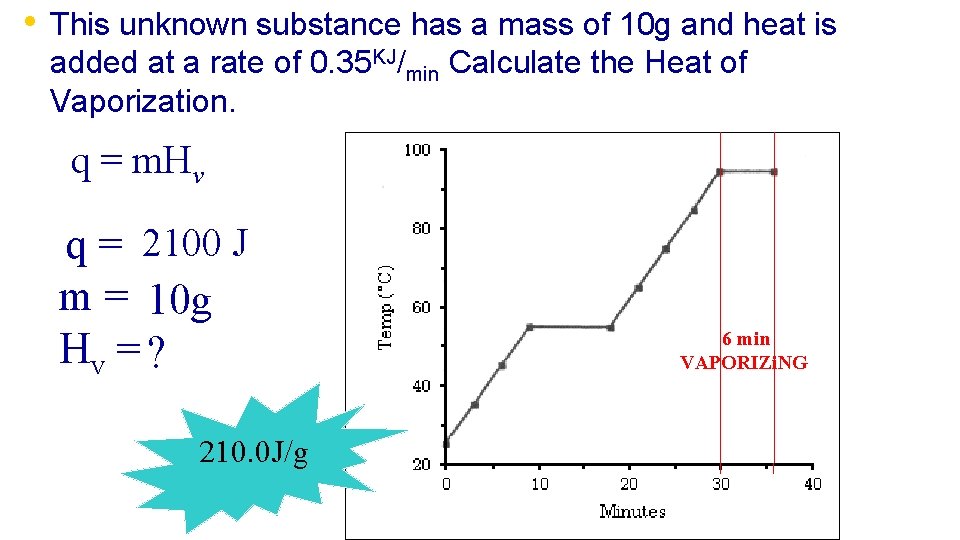
• This unknown substance has a mass of 10 g and heat is added at a rate of 0. 35 KJ/min Calculate the Heat of Vaporization. q = m. Hv q = 2100 J m = 10 g Hv = ? 210. 0 J/g 6 min VAPORIZi. NG

• Mmmmm I want to make a pizza and I pre-heat the oven to 475 o. F (~246 o. C). As I get ready to put the pizza in the oven, I realize that I’ve left an Aluminum cookie sheet in there. Oops! • Earlier I washing dishes, and I left the sink full of • water, now its gross and cold. I put ovenmits on and take out the hot cookie sheet, and put it into the water. Then put the pizza in the oven to cook. Finally I go to take care of the cookie sheet, and realize that the water is really warm, and I don’t need ovenmits to touch the aluminum pan. Hmmmmm what happened?

• How hot was the Aluminum pan in the oven? • What was the approximate temperature of the “cold” water in the sink? • What happened to the temps of the pan and the water when they came into contact with each other? Is this change the same? • Where did the Energy come from that raised the temperature of the water?

• How hot was the Aluminum pan in the oven? • – The same temp as the oven 475 o. F (~246 o. C). What was the approximate temperature of the “cold” water in the sink? If it sat for a while, probably 20 o. C room temp. • What happened to the temps of the pan and the water when they came into contact with each other? The temp of the pan went down and the temp of the water went up. Is this change the same? NO! The temp of the water may have gone up to 24 o. C (ΔT of water = 4 o. C) while the temp of the pan came down to 24 o. C. (ΔT of pan = 222 o. C) • Where did the Energy come from that raised the temperature of the water? The Energy came from the pan!

The End
 Heat fusion formula
Heat fusion formula Specific heat capacity table pdf
Specific heat capacity table pdf Unit of latent heat
Unit of latent heat The energy needed to maintain life-sustaining activities
The energy needed to maintain life-sustaining activities How is specific gravity calculated
How is specific gravity calculated Specific volume to specific gravity
Specific volume to specific gravity How are thermal energy and temperature different
How are thermal energy and temperature different The amount of energy required
The amount of energy required Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Unit of specific heat
Unit of specific heat Specific heat of sugar syrup
Specific heat of sugar syrup Specific heat capacity of ice cream
Specific heat capacity of ice cream Final temperature formula
Final temperature formula Specific heat equation units
Specific heat equation units How to find the specific heat capacity
How to find the specific heat capacity