SOMERSM N COORDNATON COMP Isomers do not necessarily


![[ML 4 X 2] Type Octahedral Complexes trans-[Co(NH 3)4 Cl 2]+ cis-[Co(NH 3)4 Cl [ML 4 X 2] Type Octahedral Complexes trans-[Co(NH 3)4 Cl 2]+ cis-[Co(NH 3)4 Cl](https://slidetodoc.com/presentation_image_h2/72e45b919ed5df75db1cde651dea3bc1/image-13.jpg)
![[ML 3 X 3] Type with Octahedral Complexr MERIDIONAL mer-[Co(NH 3)3(NO 2)3] FACİAL fac-[Co(NH [ML 3 X 3] Type with Octahedral Complexr MERIDIONAL mer-[Co(NH 3)3(NO 2)3] FACİAL fac-[Co(NH](https://slidetodoc.com/presentation_image_h2/72e45b919ed5df75db1cde651dea3bc1/image-14.jpg)
- Slides: 16

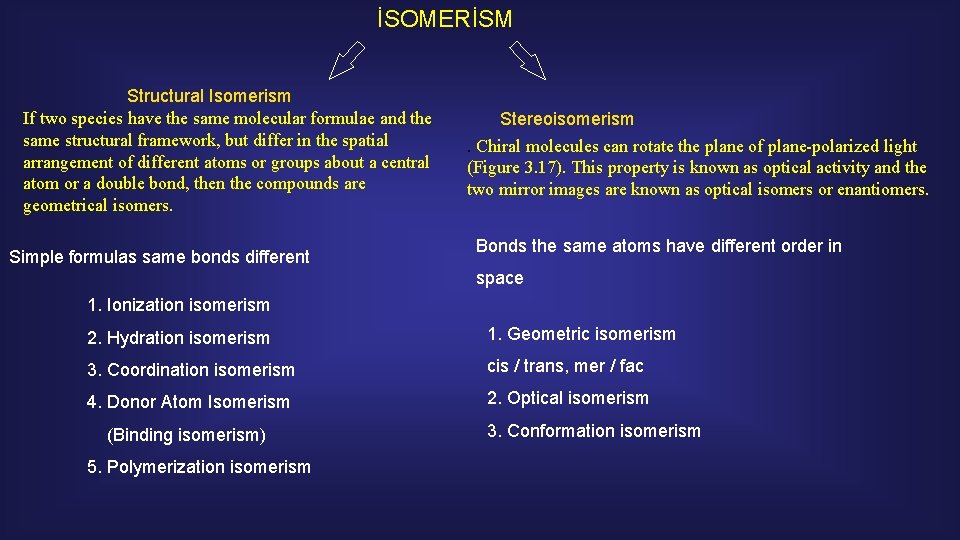
İSOMERİSM İN COORDİNATİON COMP

Isomers do not necessarily share similar chemical or physical properties. Two main forms of isomerism are structural or constitutional isomerism, in which bonds between the atoms differ; and stereoisomerism or spatial isomerism, in which the bonds are the same but the relative positions of the atoms differ.

İSOMERİSM Structural Isomerism If two species have the same molecular formulae and the same structural framework, but differ in the spatial arrangement of different atoms or groups about a central atom or a double bond, then the compounds are geometrical isomers. Simple formulas same bonds different Stereoisomerism. Chiral molecules can rotate the plane of plane-polarized light (Figure 3. 17). This property is known as optical activity and the two mirror images are known as optical isomers or enantiomers. Bonds the same atoms have different order in space 1. Ionization isomerism 2. Hydration isomerism 1. Geometric isomerism 3. Coordination isomerism cis / trans, mer / fac 4. Donor Atom Isomerism 2. Optical isomerism (Binding isomerism) 5. Polymerization isomerism 3. Conformation isomerism

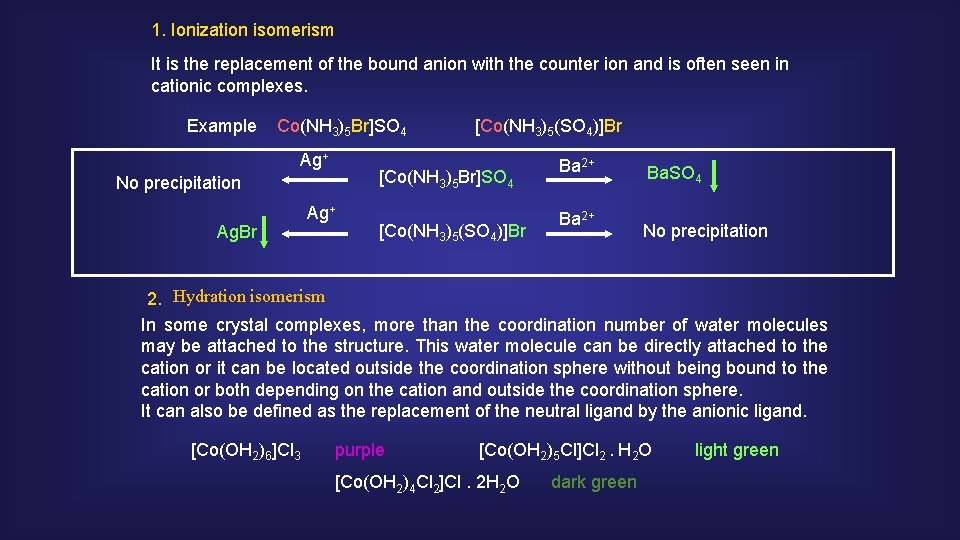
1. Ionization isomerism It is the replacement of the bound anion with the counter ion and is often seen in cationic complexes. Example Co(NH 3)5 Br]SO 4 Ag+ [Co(NH 3)5 Br]SO 4 No precipitation Ag. Br [Co(NH 3)5(SO 4)]Br Ag+ [Co(NH 3)5(SO 4)]Br Ba 2+ Ba. SO 4 No precipitation 2. Hydration isomerism In some crystal complexes, more than the coordination number of water molecules may be attached to the structure. This water molecule can be directly attached to the cation or it can be located outside the coordination sphere without being bound to the cation or both depending on the cation and outside the coordination sphere. It can also be defined as the replacement of the neutral ligand by the anionic ligand. [Co(OH 2)6]Cl 3 purple [Co(OH 2)5 Cl]Cl 2. H 2 O [Co(OH 2)4 Cl 2]Cl. 2 H 2 O dark green light green

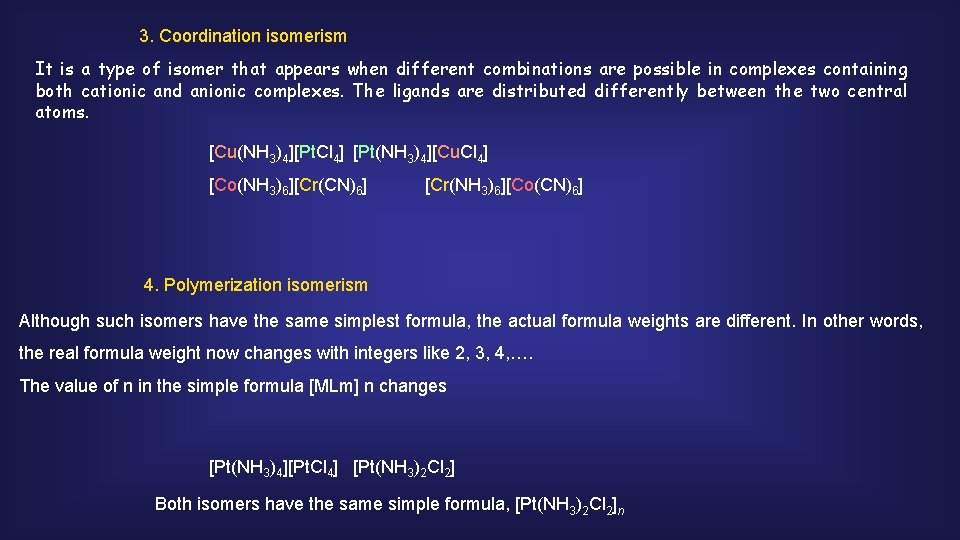
3. Coordination isomerism It is a type of isomer that appears when different combinations are possible in complexes containing both cationic and anionic complexes. The ligands are distributed differently between the two central atoms. [Cu(NH 3)4][Pt. Cl 4] [Pt(NH 3)4][Cu. Cl 4] [Co(NH 3)6][Cr(CN)6] [Cr(NH 3)6][Co(CN)6] 4. Polymerization isomerism Although such isomers have the same simplest formula, the actual formula weights are different. In other words, the real formula weight now changes with integers like 2, 3, 4, …. The value of n in the simple formula [MLm] n changes [Pt(NH 3)4][Pt. Cl 4] [Pt(NH 3)2 Cl 2] Both isomers have the same simple formula, [Pt(NH 3)2 Cl 2]n

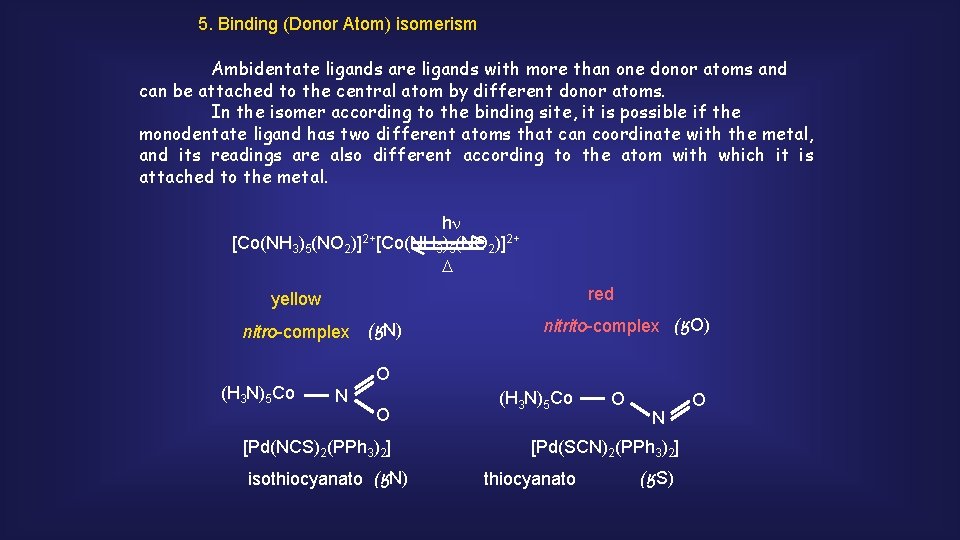
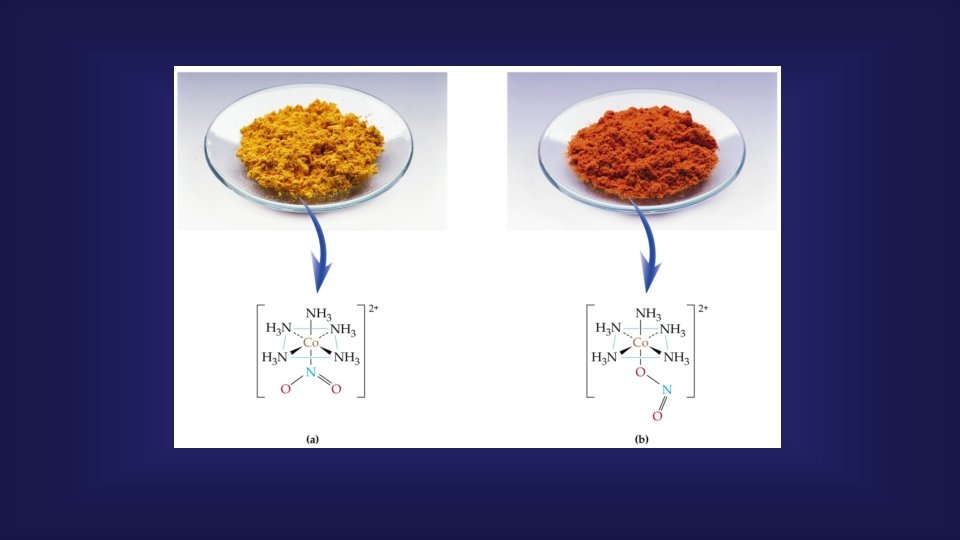
5. Binding (Donor Atom) isomerism Ambidentate ligands are ligands with more than one donor atoms and can be attached to the central atom by different donor atoms. In the isomer according to the binding site, it is possible if the monodentate ligand has two different atoms that can coordinate with the metal, and its readings are also different according to the atom with which it is attached to the metal. [Co(NH 3)5(NO 2 )]2+ [Co(NH hn 2+ 3)5(NO 2)] D red yellow nitro-complex (ӄN) (H 3 N)5 Co nitrito-complex (ӄO) O N O [Pd(NCS)2(PPh 3)2] isothiocyanato (ӄN) (H 3 N)5 Co O N [Pd(SCN)2(PPh 3)2] thiocyanato (ӄS) O


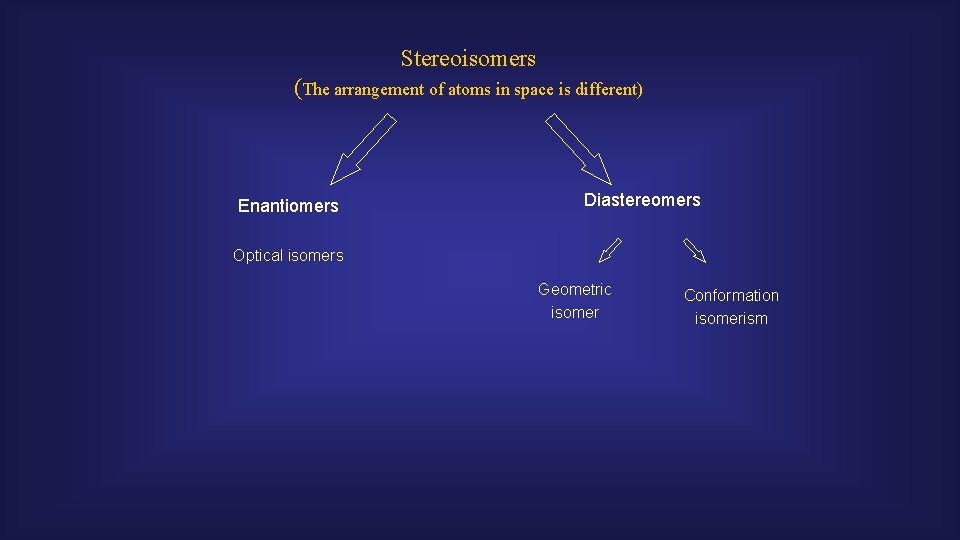
Stereoisomers (The arrangement of atoms in space is different) Enantiomers Diastereomers Optical isomers Geometric isomer Conformation isomerism

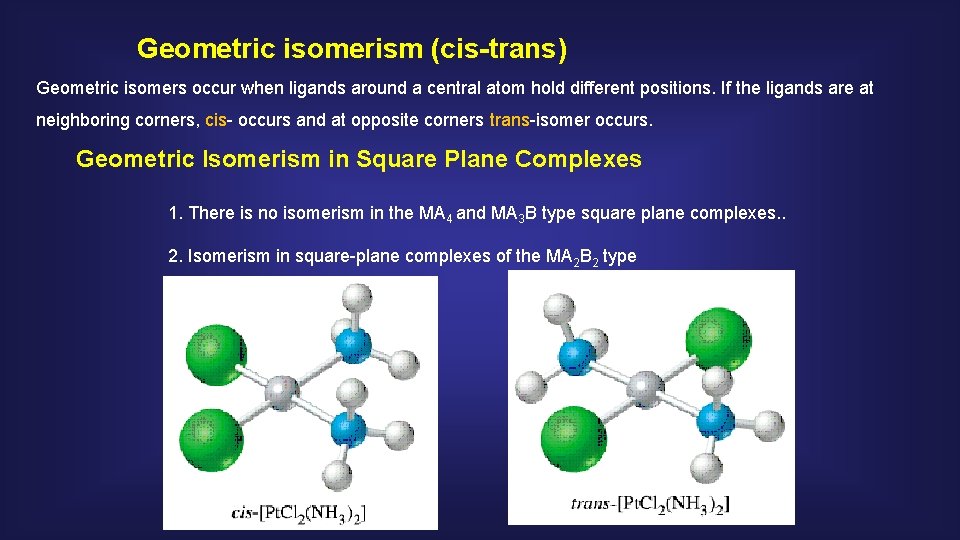
Geometric isomerism (cis-trans) Geometric isomers occur when ligands around a central atom hold different positions. If the ligands are at neighboring corners, cis- occurs and at opposite corners trans-isomer occurs. Geometric Isomerism in Square Plane Complexes 1. There is no isomerism in the MA 4 and MA 3 B type square plane complexes. . 2. Isomerism in square-plane complexes of the MA 2 B 2 type

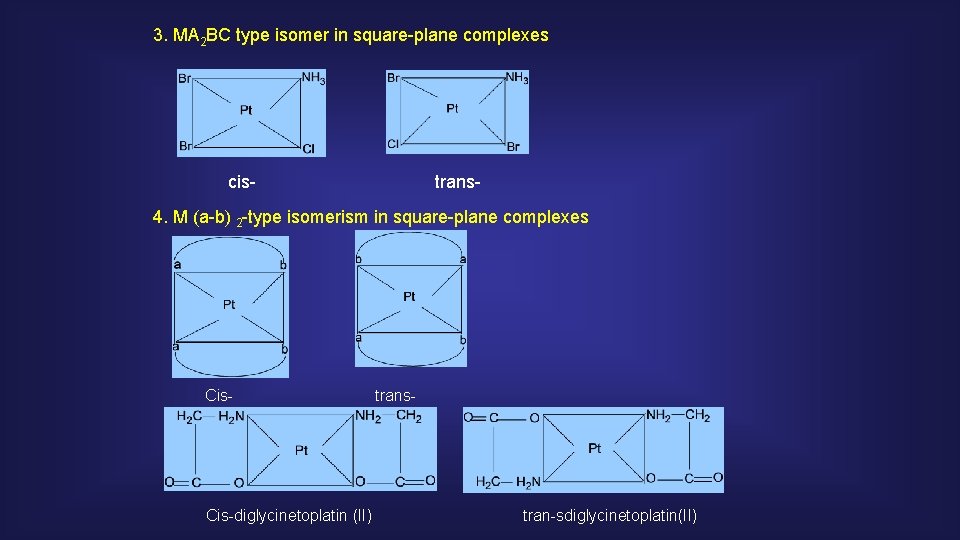
3. MA 2 BC type isomer in square-plane complexes cis- trans- 4. M (a-b) 2 -type isomerism in square-plane complexes Cis-diglycinetoplatin (II) trans- tran-sdiglycinetoplatin(II)

Geometric Isomerism in Tetrehedral Complexes Since all corners are adjacent to each other in tetrehedral complexes, the geometric isomerism is not observed in these complexes. However, asymmetric structures have optical isomers.

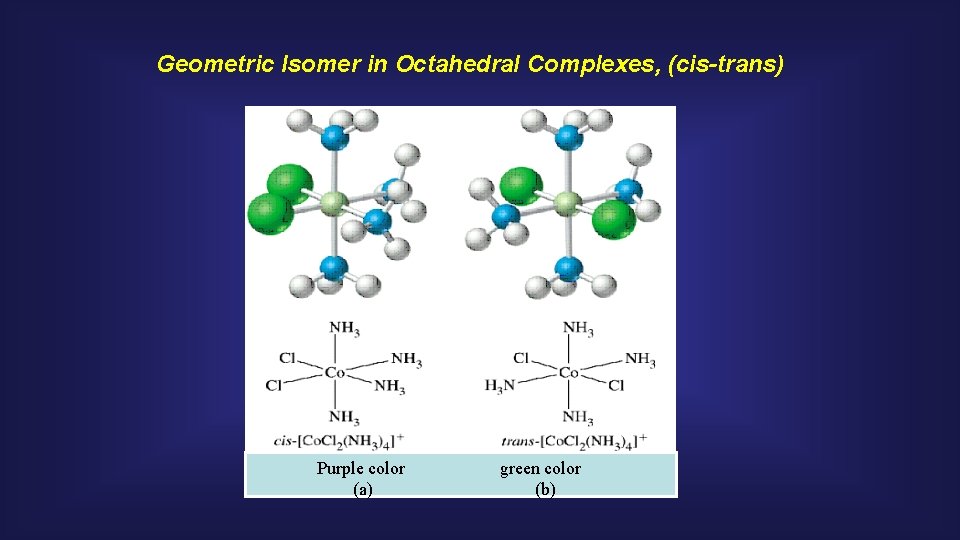
Geometric Isomer in Octahedral Complexes, (cis-trans) Purple color (a) green color (b)
![ML 4 X 2 Type Octahedral Complexes transCoNH 34 Cl 2 cisCoNH 34 Cl [ML 4 X 2] Type Octahedral Complexes trans-[Co(NH 3)4 Cl 2]+ cis-[Co(NH 3)4 Cl](https://slidetodoc.com/presentation_image_h2/72e45b919ed5df75db1cde651dea3bc1/image-13.jpg)
[ML 4 X 2] Type Octahedral Complexes trans-[Co(NH 3)4 Cl 2]+ cis-[Co(NH 3)4 Cl 2]+ green purple
![ML 3 X 3 Type with Octahedral Complexr MERIDIONAL merCoNH 33NO 23 FACİAL facCoNH [ML 3 X 3] Type with Octahedral Complexr MERIDIONAL mer-[Co(NH 3)3(NO 2)3] FACİAL fac-[Co(NH](https://slidetodoc.com/presentation_image_h2/72e45b919ed5df75db1cde651dea3bc1/image-14.jpg)
[ML 3 X 3] Type with Octahedral Complexr MERIDIONAL mer-[Co(NH 3)3(NO 2)3] FACİAL fac-[Co(NH 3)3(NO 2)3]

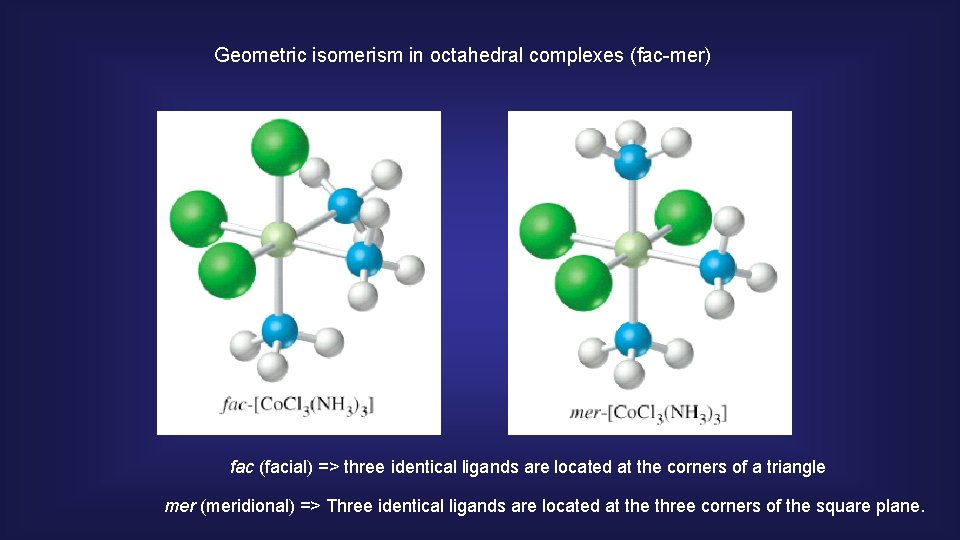
Geometric isomerism in octahedral complexes (fac-mer) fac (facial) => three identical ligands are located at the corners of a triangle mer (meridional) => Three identical ligands are located at the three corners of the square plane.

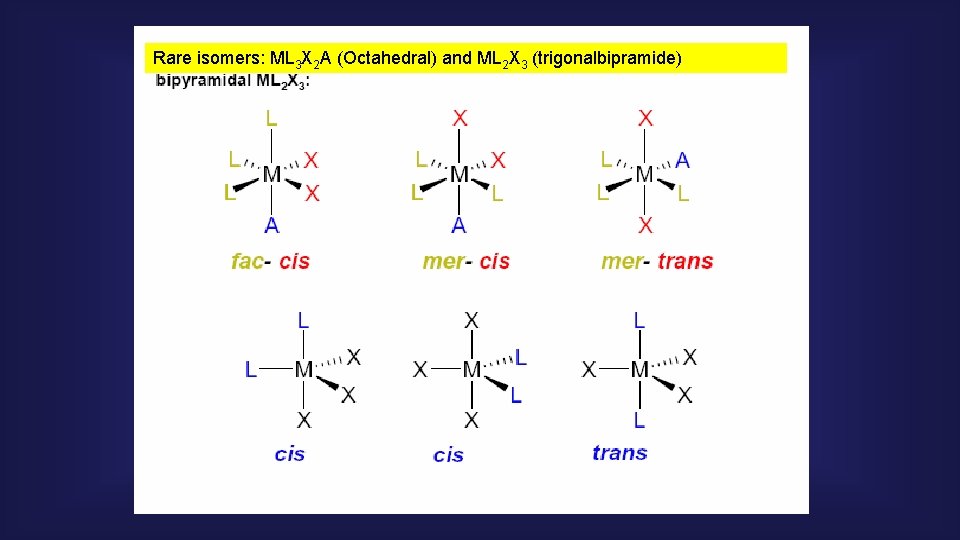
Rare isomers: ML 3 X 2 A (Octahedral) and ML 2 X 3 (trigonalbipramide)