SDSPAGE Sodium Dodecyl Sulfate Polyacrylamid Gel Electrophoresis Timothy


- Slides: 13

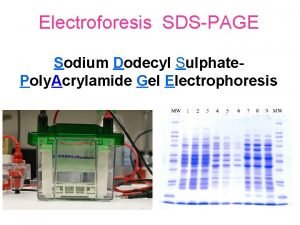
SDS-PAGE Sodium Dodecyl Sulfate Polyacrylamid Gel Electrophoresis Timothy G. Standish, Ph. D. © 1999 Timothy G. Standish

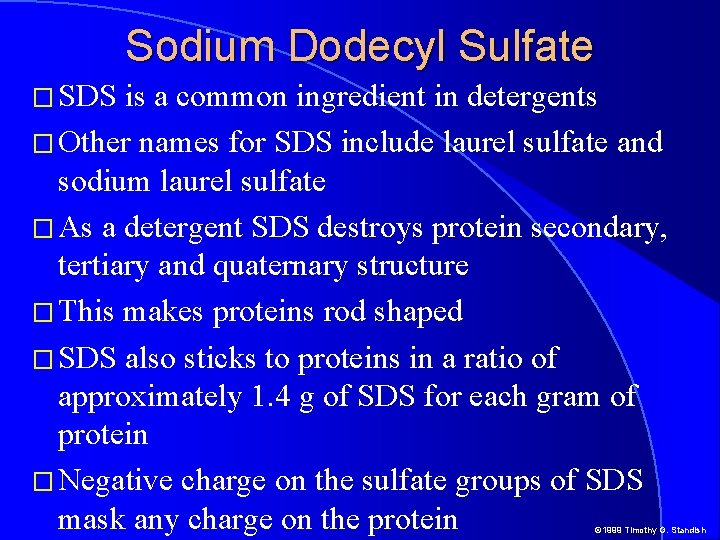
Sodium Dodecyl Sulfate � SDS is a common ingredient in detergents � Other names for SDS include laurel sulfate and sodium laurel sulfate � As a detergent SDS destroys protein secondary, tertiary and quaternary structure � This makes proteins rod shaped � SDS also sticks to proteins in a ratio of approximately 1. 4 g of SDS for each gram of protein � Negative charge on the sulfate groups of SDS mask any charge on the protein © 1999 Timothy G. Standish

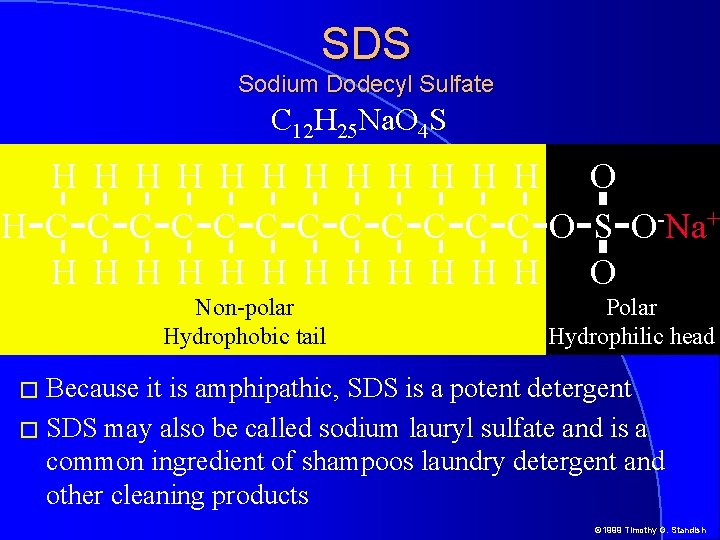
SDS Sodium Dodecyl Sulfate C 12 H 25 Na. O 4 S H H H O H-C-C-C-C-C-C-O-S-O-Na+ H H H O Non-polar Hydrophobic tail Polar Hydrophilic head � Because it is amphipathic, SDS is a potent detergent � SDS may also be called sodium lauryl sulfate and is a common ingredient of shampoos laundry detergent and other cleaning products © 1999 Timothy G. Standish

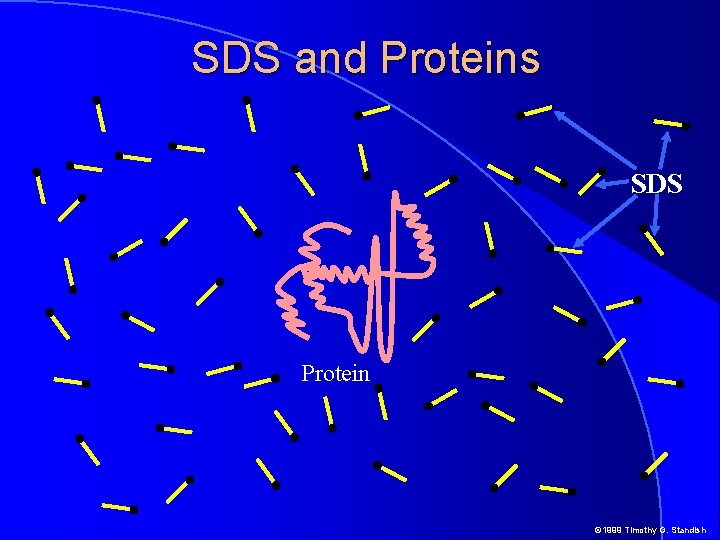
SDS and Proteins SDS Protein © 1999 Timothy G. Standish

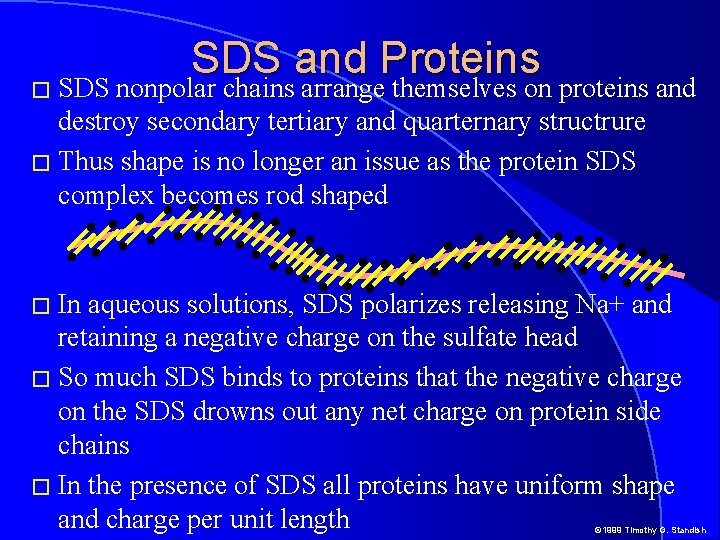
SDS and Proteins � SDS nonpolar chains arrange themselves on proteins and destroy secondary tertiary and quarternary structrure � Thus shape is no longer an issue as the protein SDS complex becomes rod shaped � In aqueous solutions, SDS polarizes releasing Na+ and retaining a negative charge on the sulfate head � So much SDS binds to proteins that the negative charge on the SDS drowns out any net charge on protein side chains � In the presence of SDS all proteins have uniform shape and charge per unit length © 1999 Timothy G. Standish

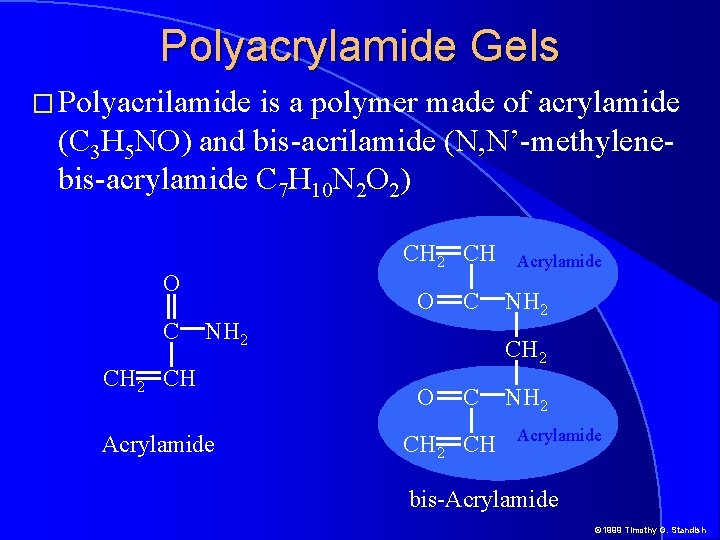
Polyacrylamide Gels � Polyacrilamide is a polymer made of acrylamide (C 3 H 5 NO) and bis-acrilamide (N, N’-methylenebis-acrylamide C 7 H 10 N 2 O 2) CH 2 CH O C NH 2 CH Acrylamide NH 2 CH 2 O C CH 2 CH NH 2 Acrylamide bis-Acrylamide © 1999 Timothy G. Standish

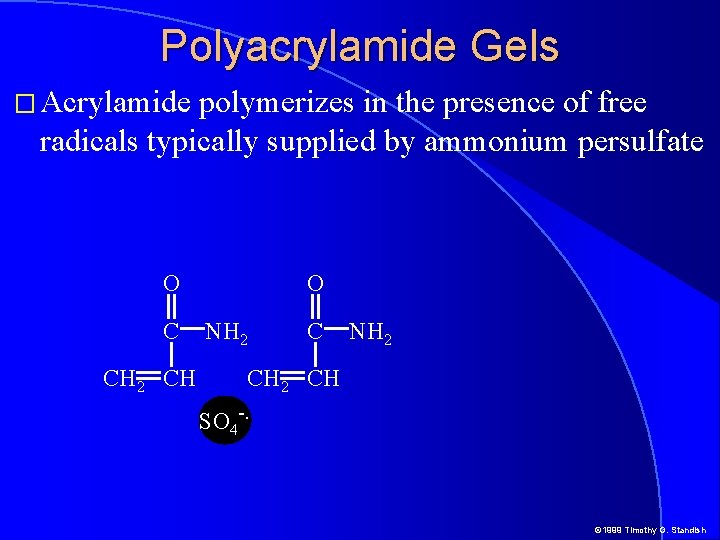
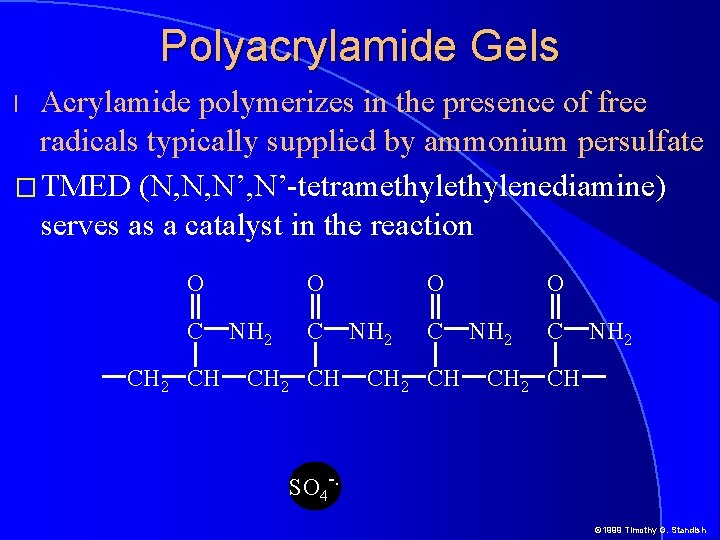
Polyacrylamide Gels � Acrylamide polymerizes in the presence of free radicals typically supplied by ammonium persulfate O C CH 2 CH O NH 2 CH SO 4 -. © 1999 Timothy G. Standish

Polyacrylamide Gels Acrylamide polymerizes in the presence of free radicals typically supplied by ammonium persulfate � TMED (N, N, N’-tetramethylenediamine) serves as a catalyst in the reaction l O C CH 2 CH O NH 2 CH 2 CH SO 4 -. © 1999 Timothy G. Standish

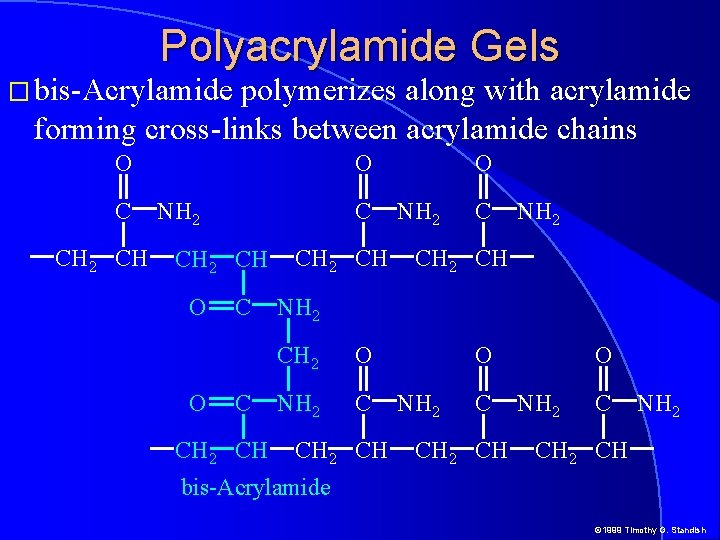
Polyacrylamide Gels � bis-Acrylamide polymerizes along with acrylamide forming cross-links between acrylamide chains O C CH 2 CH O NH 2 C CH 2 CH O O C C CH 2 CH O NH 2 CH NH 2 CH 2 O NH 2 C CH 2 CH O NH 2 CH bis-Acrylamide © 1999 Timothy G. Standish

Polyacrylamide Gels � bis-Acrylamide polymerizes along with acrylamide forming cross-links between acrylamide chains © 1999 Timothy G. Standish

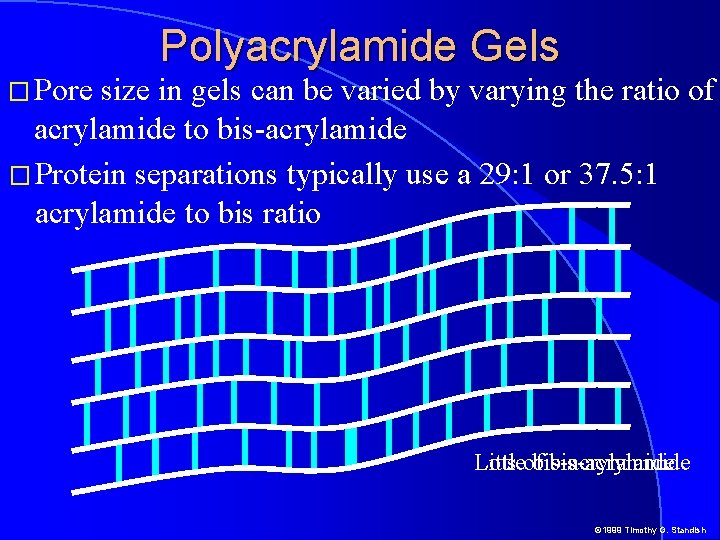
� Pore Polyacrylamide Gels size in gels can be varied by varying the ratio of acrylamide to bis-acrylamide � Protein separations typically use a 29: 1 or 37. 5: 1 acrylamide to bis ratio Lots Littleofbis-acrylamide © 1999 Timothy G. Standish

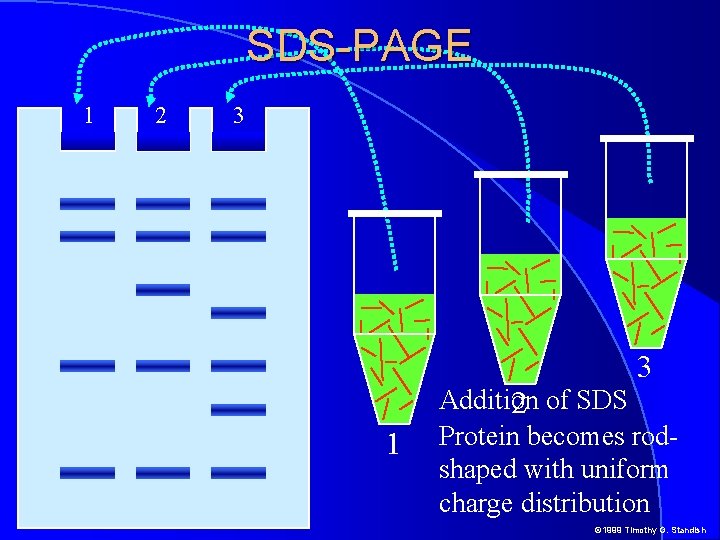
SDS-PAGE 1 2 3 3 1 Addition 2 of SDS Protein becomes rodshaped with uniform charge distribution © 1999 Timothy G. Standish

© 1999 Timothy G. Standish
 Procedure of sds page
Procedure of sds page Calcium + nitric acid balanced equation
Calcium + nitric acid balanced equation What are spectator ions
What are spectator ions Polyacrylamide gel electrophoresis (page)
Polyacrylamide gel electrophoresis (page) How to use micropipette
How to use micropipette Restriction enzymes gel electrophoresis
Restriction enzymes gel electrophoresis Electrophoresis definition
Electrophoresis definition Advantages of agarose gel electrophoresis
Advantages of agarose gel electrophoresis Anode cathode gel electrophoresis
Anode cathode gel electrophoresis Ap biology lab 6
Ap biology lab 6 Lambda electrolysis
Lambda electrolysis Gel electrophoresis result
Gel electrophoresis result Gel electrophoresis separates dna by
Gel electrophoresis separates dna by Process of gel electrophoresis
Process of gel electrophoresis