Relapsed Refractory MM Patients ASH 2015 Summary ELOQUENT2



















- Slides: 105

Relapsed / Refractory MM Patients ASH 2015 Summary

ELOQUENT-2 Update: A Phase 3, Randomized, Open-Label Study of Elotuzumab in Combination with Lenalidomide/Dexamethasone in Patients with Relapsed/Refractory Multiple Myeloma – 3 -Year Safety and Efficacy Follow-up Meletios Dimopoulos, 1 Sagar Lonial, 2 Darrell White, 3 Philippe Moreau, 4 Antonio Palumbo, 5 Jesus San Miguel, 6 Ofer Shpilberg, 7 Kenneth Anderson, 8 Sebastian Grosicki, 9 Ivan Spicka, 10 Adam Walter-Croneck, 11 Hila Magen. Nativ, 12 Maria-Victoria Mateos, 13 Andrew Belch, 14 Donna Reece, 15 Meral Beksac, 16 Eric Bleickardt, 17 Valerie Poulart, 18 Jessica Katz, 19 Anil Singhal, 20 Paul Richardson 8 1 National and Kapodistrian University of Athens, Greece; 2 Winship Cancer Institute, Emory University School of Medicine, Atlanta, USA; 3 QEII Health Science Center and Dalhousie University, Halifax, Canada; 4 University Hospital, Nantes, France; 5 A. O. U. San Giovanni Battista di Torino - Ospedale Molinette, Torino, Italy; 6 Clinical Universidad de Navarra, Pamplona, Spain; 7 Assuta Medical Centers, Tel-Aviv, Israel; 8 Dana-Farber Cancer Institute, Boston, MA; 9 Silesian Medical University, Katowice, Poland; 10 Charles University Hospital, Prague, Czech Republic; 11 Medical University of Lublin, Poland; 12 Davidoff Cancer Center, Rabin Medical Center, Petah Tikva, and Tel Aviv University, Ramat Aviv, Israel; 13 University Hospital of Salamanca. IBSAL, Salamanca, Spain; 14 Cross Cancer Institute and University of Alberta, Edmonton, Canada; 15 Princess Margaret Cancer Center, Toronto, Canada; 16 Ankara University, Ankara, Turkey; 17 Bristol-Myers Squibb, Wallingford, CT; 18 Bristol-Myers Squibb, Braine-l'Alleud, Belgium; 19 Bristol-Myers Squibb, Princeton, NJ; 20 Abb. Vie Biotherapeutics Inc. (ABR), Redwood City, CA American Society of Hematology (ASH) Annual Meeting & Exposition; December 5– 8, 2015; Orlando, Florida Dimopoulos M, ASH 2015 Abst 28

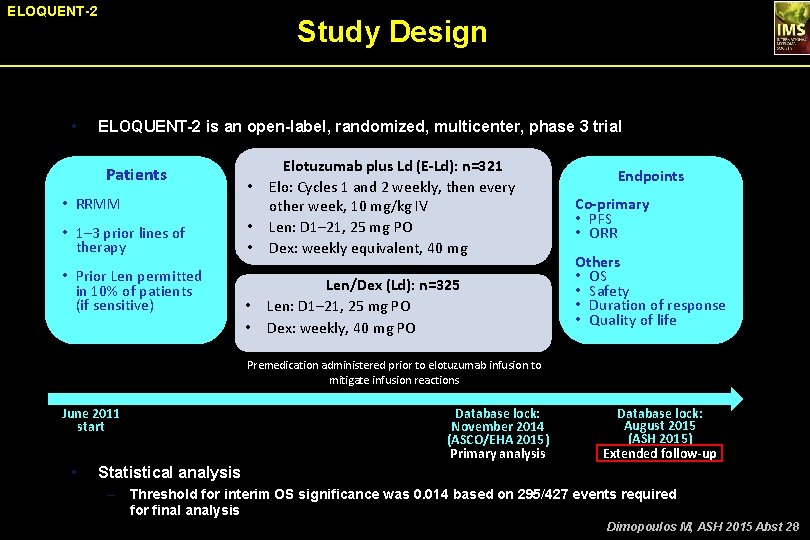
ELOQUENT-2 • Study Design ELOQUENT-2 is an open-label, randomized, multicenter, phase 3 trial Patients • RRMM • 1– 3 prior lines of therapy • Prior Len permitted in 10% of patients (if sensitive) • • Elotuzumab plus Ld (E-Ld): n=321 Elo: Cycles 1 and 2 weekly, then every other week, 10 mg/kg IV Len: D 1– 21, 25 mg PO Dex: weekly equivalent, 40 mg • • Len/Dex (Ld): n=325 Len: D 1– 21, 25 mg PO Dex: weekly, 40 mg PO • Endpoints Co-primary • PFS • ORR Others • OS • Safety • Duration of response • Quality of life Premedication administered prior to elotuzumab infusion to mitigate infusion reactions June 2011 start • Database lock: November 2014 (ASCO/EHA 2015) Primary analysis Database lock: August 2015 (ASH 2015) Extended follow-up Statistical analysis – Threshold for interim OS significance was 0. 014 based on 295/427 events required for final analysis Dimopoulos M, ASH 2015 Abst 28

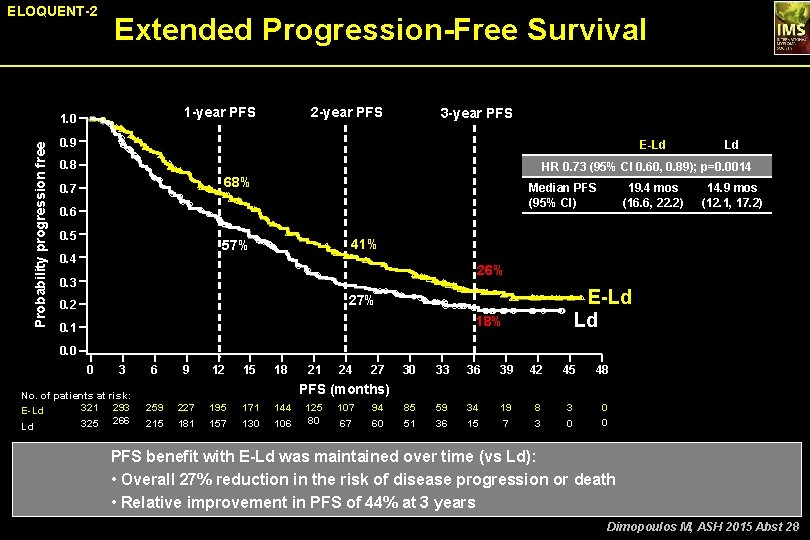
ELOQUENT-2 Extended Progression-Free Survival 1 -year PFS Probability progression free 1. 0 2 -year PFS 3 -year PFS 0. 9 E-Ld 0. 8 Ld HR 0. 73 (95% CI 0. 60, 0. 89); p=0. 0014 68% 0. 7 Median PFS (95% CI) 0. 6 0. 5 14. 9 mos (12. 1, 17. 2) 41% 57% 0. 4 19. 4 mos (16. 6, 22. 2) 26% 0. 3 E-Ld Ld 27% 0. 2 18% 0. 1 0. 0 0 3 No. of patients at risk: 321 293 E-Ld 325 266 Ld 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 85 51 59 36 34 15 19 7 8 3 3 0 0 0 PFS (months) 259 215 227 181 195 157 171 130 144 106 125 80 107 67 94 60 PFS benefit with E-Ld was maintained over time (vs Ld): • Overall 27% reduction in the risk of disease progression or death • Relative improvement in PFS of 44% at 3 years Dimopoulos M, ASH 2015 Abst 28

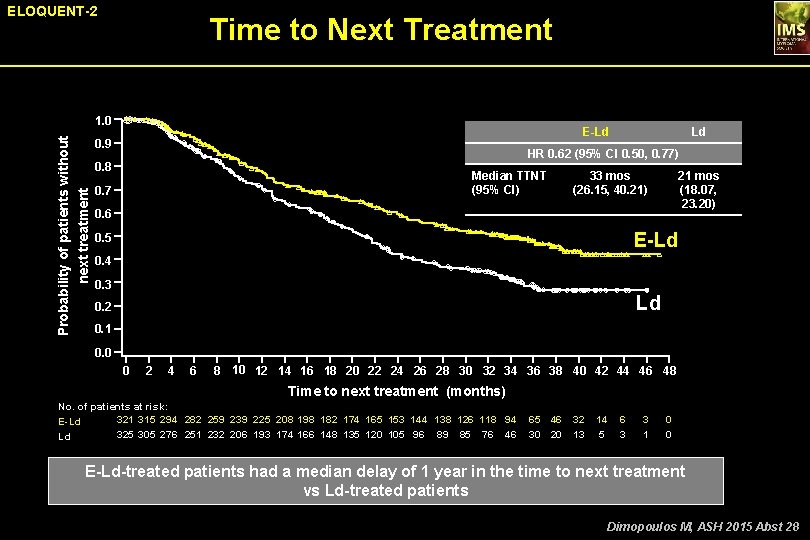
ELOQUENT-2 Time to Next Treatment Probability of patients without next treatment 1. 0 E-Ld 0. 9 Ld HR 0. 62 (95% CI 0. 50, 0. 77) 0. 8 Median TTNT (95% CI) 0. 7 33 mos (26. 15, 40. 21) 21 mos (18. 07, 23. 20) 0. 6 E-Ld 0. 5 0. 4 0. 3 Ld 0. 2 0. 1 0. 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46 48 Time to next treatment (months) No. of patients at risk: 321 315 294 282 259 239 225 208 198 182 174 165 153 144 138 126 118 94 E-Ld 325 305 276 251 232 206 193 174 166 148 135 120 105 96 89 85 76 46 Ld 65 46 32 30 20 13 14 5 6 3 3 1 0 0 E-Ld-treated patients had a median delay of 1 year in the time to next treatment vs Ld-treated patients Dimopoulos M, ASH 2015 Abst 28

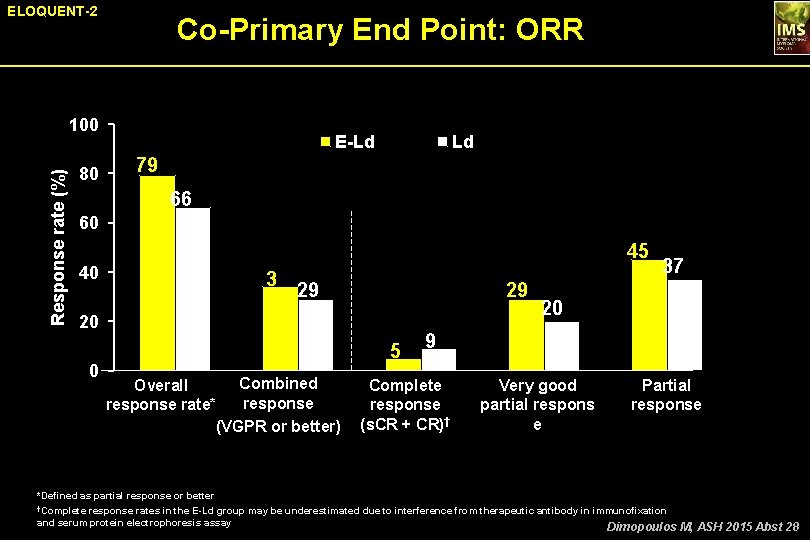
ELOQUENT-2 Co-Primary End Point: ORR Response rate (%) 100 80 E-Ld Ld 79 66 60 45 40 20 0 3 4 29 29 5 Combined Overall response rate* (VGPR or better) 37 20 9 Complete response (s. CR + CR)† Very good partial respons e Partial response *Defined as partial response or better †Complete response rates in the E-Ld group may be underestimated due to interference from therapeutic antibody in immunofixation and serum protein electrophoresis assay Dimopoulos M, ASH 2015 Abst 28

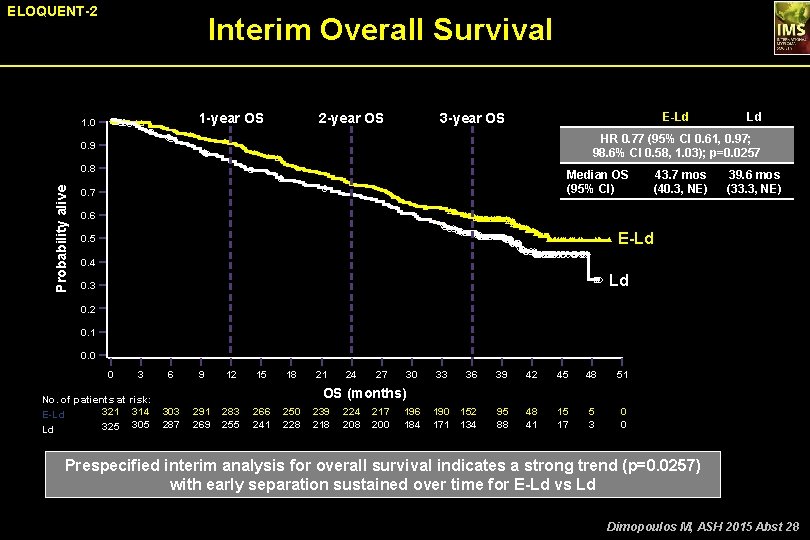
ELOQUENT-2 Interim Overall Survival 1 -year OS 1. 0 2 -year OS E-Ld 3 -year OS HR 0. 77 (95% CI 0. 61, 0. 97; 98. 6% CI 0. 58, 1. 03); p=0. 0257 0. 9 0. 8 Probability alive Ld Median OS (95% CI) 0. 7 43. 7 mos (40. 3, NE) 39. 6 mos (33. 3, NE) 0. 6 E-Ld 0. 5 0. 4 Ld 0. 3 0. 2 0. 1 0. 0 0 3 No. of patients at risk: 321 314 E-Ld 325 305 Ld 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 51 190 152 171 134 95 88 48 41 15 17 5 3 0 0 OS (months) 303 287 291 269 283 255 266 241 250 228 239 218 224 208 217 200 196 184 Prespecified interim analysis for overall survival indicates a strong trend (p=0. 0257) with early separation sustained over time for E-Ld vs Ld Dimopoulos M, ASH 2015 Abst 28

ELOQUENT-2 • Summary Elotuzumab, a novel immunostimulatory monoclonal antibody, in combination with Ld, demonstrated a durable and clinically relevant improvement in PFS and ORR – Extended follow-up demonstrated a 27% reduction in the risk of progression or death compared with Ld alone (HR 0. 73; p=0. 0014) – 38% fewer patients in the E-Ld vs Ld arm started a subsequent line of therapy during the follow-up period – PFS benefits with E-Ld were consistent across key subgroups • Interim overall survival analysis demonstrated a strong trend in favor of E-Ld vs Ld (HR 0. 77; p=0. 0257) • Updated safety and tolerability data are consistent with previous findings, confirming that there is minimal incremental toxicity associated with the addition of elotuzumab to Ld • FDA recently granted approval for the use of elotuzumab in combination with Ld in patients with multiple myeloma who have received one to three prior therapies Dimopoulos M, ASH 2015 Abst 28

Elotuzumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma: 2 -year follow-up Antonio Palumbo, 1 Massimo Offidani, 2 Brigitte Pégourie, 3 Javier De La Rubia, 4 Laurent Garderet, 5 Kamel Laribi, 6 Alberto Bosi, 7 Roberto Marasca, 8 Jacob Laubach, 9 Ann Mohrbacher, 10 Angelo Michele Carella, 11 Anil K Singhal, 12 Mark Lynch, 13 Ying-Ming Jou, 14 Andrzej Jakubowiak 15 Palumbo A, ASH 2015 Abst 510

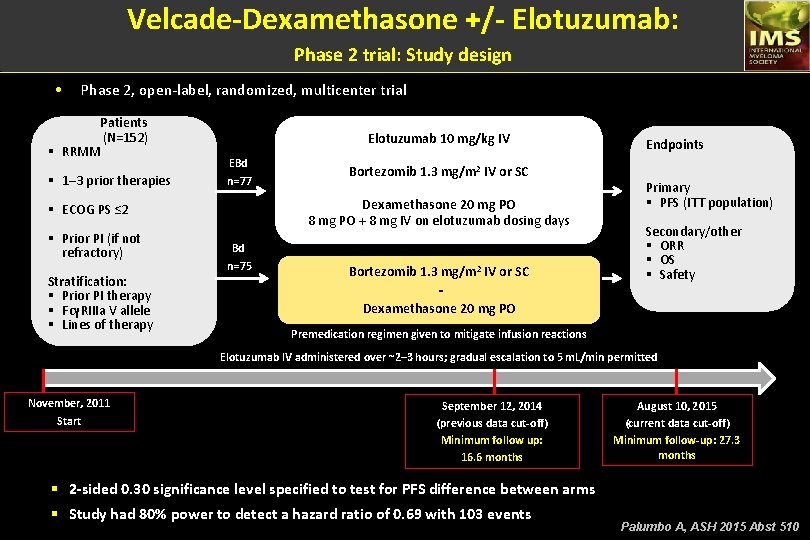
Velcade-Dexamethasone +/- Elotuzumab: Phase 2 trial: Study design • Phase 2, open-label, randomized, multicenter trial Patients (N=152) § RRMM § 1– 3 prior therapies Elotuzumab 10 mg/kg IV EBd n=77 Dexamethasone 20 mg PO 8 mg PO + 8 mg IV on elotuzumab dosing days § ECOG PS ≤ 2 § Prior PI (if not refractory) Stratification: § Prior PI therapy § FcγRIIIa V allele § Lines of therapy Bortezomib 1. 3 mg/m 2 IV or SC Bd n=75 Bortezomib 1. 3 mg/m 2 IV or SC Dexamethasone 20 mg PO Endpoints Primary § PFS (ITT population) Secondary/other § ORR § OS § Safety Premedication regimen given to mitigate infusion reactions Elotuzumab IV administered over ~2– 3 hours; gradual escalation to 5 m. L/min permitted November, 2011 Start September 12, 2014 (previous data cut-off) Minimum follow up: 16. 6 months August 10, 2015 (current data cut-off) Minimum follow-up: 27. 3 months § 2 -sided 0. 30 significance level specified to test for PFS difference between arms § Study had 80% power to detect a hazard ratio of 0. 69 with 103 events Palumbo A, ASH 2015 Abst 510

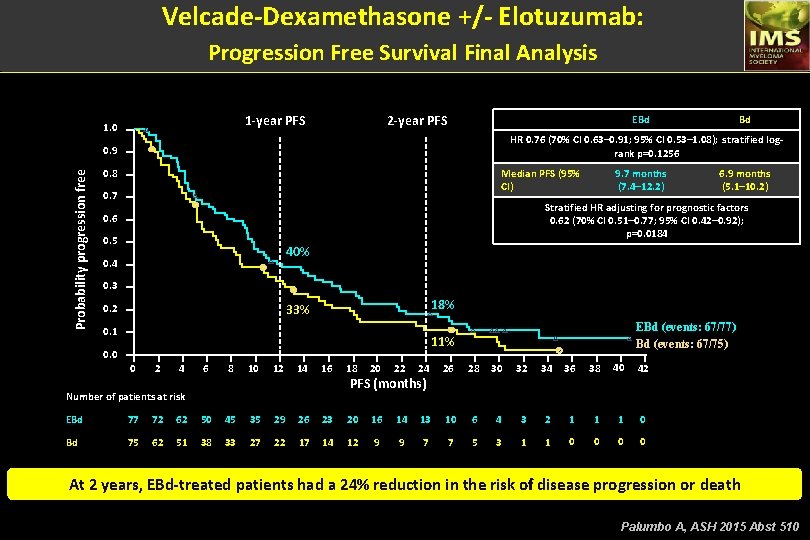
Velcade-Dexamethasone +/- Elotuzumab: Progression Free Survival Final Analysis 1 -year PFS 1. 0 2 -year PFS EBd HR 0. 76 (70% CI 0. 63– 0. 91; 95% CI 0. 53– 1. 08); stratified logrank p=0. 1256 0. 9 Probability progression free Bd 0. 8 Median PFS (95% CI) 0. 7 9. 7 months (7. 4– 12. 2) 6. 9 months (5. 1– 10. 2) Stratified HR adjusting for prognostic factors 0. 62 (70% CI 0. 51– 0. 77; 95% CI 0. 42– 0. 92); p=0. 0184 0. 6 0. 5 40% 0. 4 0. 3 18% 33% 0. 2 0. 1 EBd (events: 67/77) Bd (events: 67/75) 11% 0. 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 PFS (months) Number of patients at risk EBd 77 72 62 50 45 35 29 26 23 20 16 14 13 10 6 4 3 2 1 1 1 0 Bd 75 62 51 38 33 27 22 17 14 12 9 9 7 7 5 3 1 1 0 0 At 2 years, EBd-treated patients had a 24% reduction in the risk of disease progression or death Palumbo A, ASH 2015 Abst 510

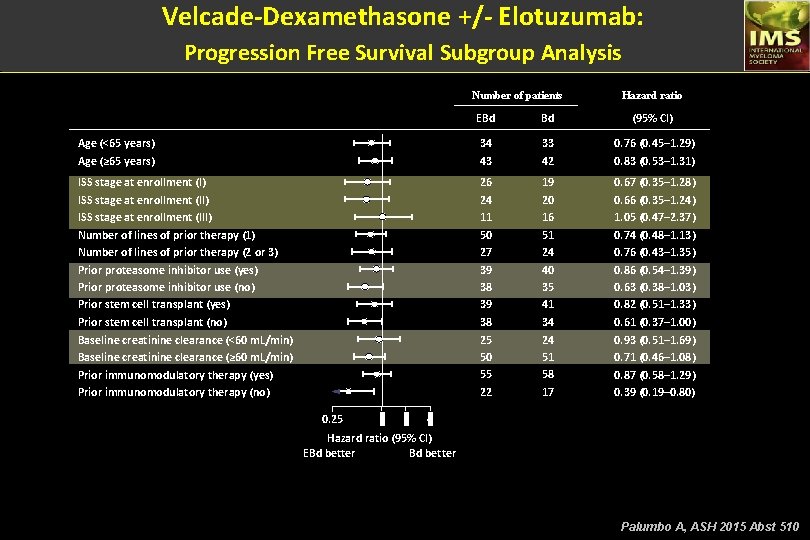
Velcade-Dexamethasone +/- Elotuzumab: Progression Free Survival Subgroup Analysis Number of patients Hazard ratio EBd Bd (95% CI) Age (<65 years) Age (≥ 65 years) 34 43 33 42 0. 76 (0. 45– 1. 29) 0. 83 (0. 53– 1. 31) ISS stage at enrollment (II) ISS stage at enrollment (III) Number of lines of prior therapy (1) Number of lines of prior therapy (2 or 3) Prior proteasome inhibitor use (yes) Prior proteasome inhibitor use (no) Prior stem cell transplant (yes) Prior stem cell transplant (no) Baseline creatinine clearance (<60 m. L/min) Baseline creatinine clearance (≥ 60 m. L/min) Prior immunomodulatory therapy (yes) Prior immunomodulatory therapy (no) 26 24 11 50 27 39 38 25 50 55 22 19 20 16 51 24 40 35 41 34 24 51 58 17 0. 67 (0. 35– 1. 28) 0. 66 (0. 35– 1. 24) 1. 05 (0. 47– 2. 37) 0. 74 (0. 48– 1. 13) 0. 76 (0. 43– 1. 35) 0. 86 (0. 54– 1. 39) 0. 63 (0. 38– 1. 03) 0. 82 (0. 51– 1. 33) 0. 61 (0. 37– 1. 00) 0. 93 (0. 51– 1. 69) 0. 71 (0. 46– 1. 08) 0. 87 (0. 58– 1. 29) 0. 39 (0. 19– 0. 80) 0. 25 1 2 4 Hazard ratio (95% CI) EBd better Palumbo A, ASH 2015 Abst 510

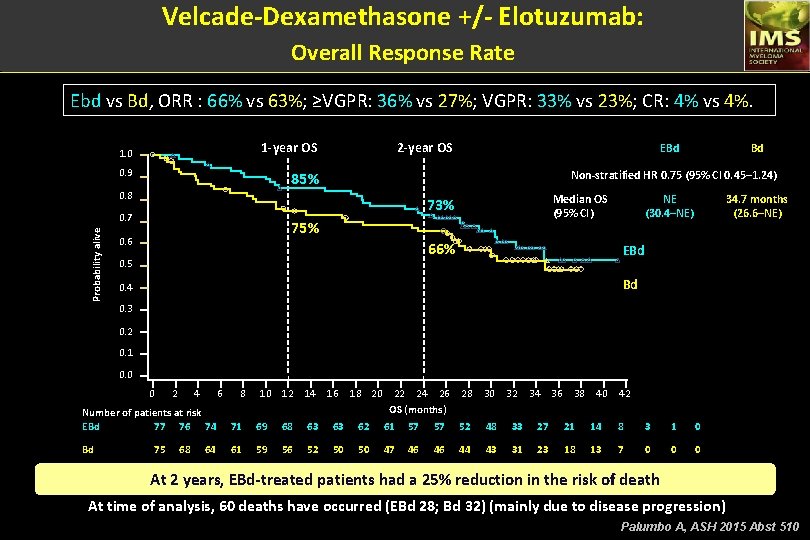
Velcade-Dexamethasone +/- Elotuzumab: Overall Response Rate Ebd vs Bd, ORR : 66% vs 63%; ≥VGPR: 36% vs 27%; VGPR: 33% vs 23%; CR: 4% vs 4%. 2 -year OS 1. 0 0. 9 Median OS (95% CI) 73% 0. 7 75% 0. 6 Bd Non-stratified HR 0. 75 (95% CI 0. 45– 1. 24) 85% 0. 8 Probability alive EBd 66% 0. 5 NE (30. 4–NE) 34. 7 months (26. 6–NE) EBd Bd 0. 4 0. 3 0. 2 0. 1 0. 0 0 2 4 6 8 1 0 1 2 1 4 1 6 1 8 20 22 24 26 OS (months) 28 30 32 34 36 38 40 42 Number of patients at risk EBd 77 76 74 71 69 68 63 63 62 61 57 57 52 48 33 27 21 14 8 3 1 0 Bd 61 59 56 52 50 50 47 46 46 44 43 31 23 18 13 7 0 0 0 75 68 64 At 2 years, EBd-treated patients had a 25% reduction in the risk of death At time of analysis, 60 deaths have occurred (EBd 28; Bd 32) (mainly due to disease progression) Palumbo A, ASH 2015 Abst 510

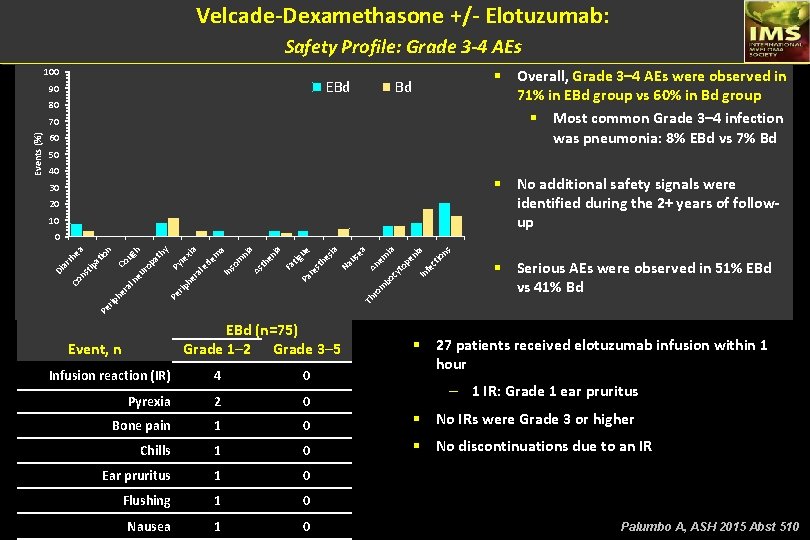
Velcade-Dexamethasone +/- Elotuzumab: Safety Profile: Grade 3 -4 AEs 100 EBd 90 § Overall, Grade 3– 4 AEs were observed in 71% in EBd group vs 60% in Bd group § Most common Grade 3– 4 infection was pneumonia: 8% EBd vs 7% Bd Bd 80 60 50 40 § No additional safety signals were identified during the 2+ years of followup 30 20 10 An em ia cy to pe ni a In fe ct io ns m bo us ea Na sia he re st tig ue Pa ia Fa en th ni a As In de so m m a xia l e ra he ro rip Th Pe ra § Serious AEs were observed in 51% EBd vs 41% Bd Pe rip he re pa l n Co eu ro Co Py th y h ug io at tip ns ar rh ea n 0 Di Events (%) 70 EBd (n=75) Grade 1─2 Grade 3─5 Event, n § 27 patients received elotuzumab infusion within 1 hour Infusion reaction (IR) 4 0 Pyrexia 2 0 Bone pain 1 0 § No IRs were Grade 3 or higher Chills 1 0 § No discontinuations due to an IR Ear pruritus 1 0 Flushing 1 0 Nausea 1 0 – 1 IR: Grade 1 ear pruritus Palumbo A, ASH 2015 Abst 510

Velcade-Dexamethasone +/- Elotuzumab: Summary • At 2 year follow-up EBd continues to show durable efficacy (PFS) vs Bd alone – 24% reduction in risk of disease progression/death – 1 year PFS: 40% for EBd vs 33% for Bd • In overall survival analysis, the trend is in favor of EBd – 25% reduction in risk of death – 1 year OS: 85% EBd vs 75% Bd • Safety profile is consistent with larger elotuzumab phase 3 clinical trials and comparable with Bd alone – Serious AEs observed in 51% EBd vs 41% Bd – Discontinuation was study drug-related in 24% EBd vs 23% EBd – Low rate of infusion reactions observed : 4% Grade 1– 2 Palumbo A, ASH 2015 Abst 510

Ixazomib, an Oral Proteasome Inhibitor, in Combination with Lenalidomide and Dexamethasone (IRd), Significantly Extends Progression-Free Survival for Patients with Relapsed and/or Refractory Multiple Myeloma: The Phase 3 TOURMALINE-MM 1 Study (NCT 01564537) • Philippe Moreau, 1 Tamás Masszi, 2 Norbert Grzasko, 3 Nizar J. Bahlis, 4 Markus Hansson, 5 Ludek Pour, 6 Irwindeep Sandhu, 7 Peter Ganly, 8 Bartrum W. Baker, 9 Sharon Jackson, 10 Anne-Marie Stoppa, 11 David Simpson, 12 Peter Gimsing, 13 Antonio Palumbo, 14 Laurent Garderet, 15 Michele Cavo, 16 Shaji Kumar, 17 Cyrille Touzeau, 1 Francis K. Buadi, 17 Jacob P. Laubach, 18 Deborah Berg, 19 Jianchang Lin, 19 Alessandra Di Bacco, 19 Ai-Min Hui, 19 Paul G. Richardson 18 1 University Hospital Hôtel Dieu, Nantes, France; 2 St. István and St. László Hospital of Budapest, Hungary; 3 Medical University of Lublin and St. John's Cancer Center, Lublin, Poland; 4 Southern Alberta Cancer Research Institute, University of Calgary, Alberta, Canada; 5 Skåne University Hospital, Lund University, Lund, Sweden; 6 University Hospital Brno, Czech Republic; 7 University of Alberta, Edmonton, Canada; 8 Christchurch Hospital, Christchurch, New Zealand; 9 Palmerston North Hospital, Palmerston North, New Zealand; 10 Middlemore Hospital, Auckland, New Zealand; 11 Institut Paoli-Calmettes, Marseille, France; 12 North Shore Hospital, Auckland, New Zealand; 13 University Hospital Rigshospitalet, Copenhagen, Denmark; 14 University of Torino, Italy; 15 Hôpital Saint Antoine, Paris, France; 16 Bologna University School of Medicine, Bologna, Italy; 17 Mayo Clinic, Rochester, MN; 18 Dana-Farber Cancer Institute, Boston, MA; 19 Millennium Pharmaceuticals, Inc. , Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. Moreau P, ASH 2015 Abst 727

TOURMALINE-MM 1: Phase 3 study of weekly oral ixazomib plus lenalidomide-dexamethasone Global, double-blind, randomized, placebo-controlled study design Ixazomib + Lenalidomide + Dexamethasone Ixazomib: 4 mg on days 1, 8, and 15 Lenalidomide: 25 mg* on days 1 -21 Dexamethasone: 40 mg on days 1, 8, 15, 22 Randomization N=722 1: 1 Repeat every 28 days until progression, or unacceptable toxicity Stratification: • Prior therapy: 1 vs 2 or 3 • ISS: I or II vs III • PI exposure: yes vs no Placebo + Lenalidomide + Dexamethasone Placebo: on days 1, 8, and 15 Lenalidomide: 25 mg* on days 1 -21 Dexamethasone: 40 mg on days 1, 8, 15, 22 Primary endpoint: • PFS Key secondary endpoints: • OS in patients with del(17 p) Response and progression (IMWG 2011 criteria 1) assessed by an independent review committee (IRC) blinded to both treatment and investigator assessment *10 mg for patients with creatinine clearance ≤ 60 or ≤ 50 m. L/min, depending on local label/practice 1. Rajkumar S, et al. Blood 2011; 117: 4691– 5. Moreau P, ASH 2015 Abst 727

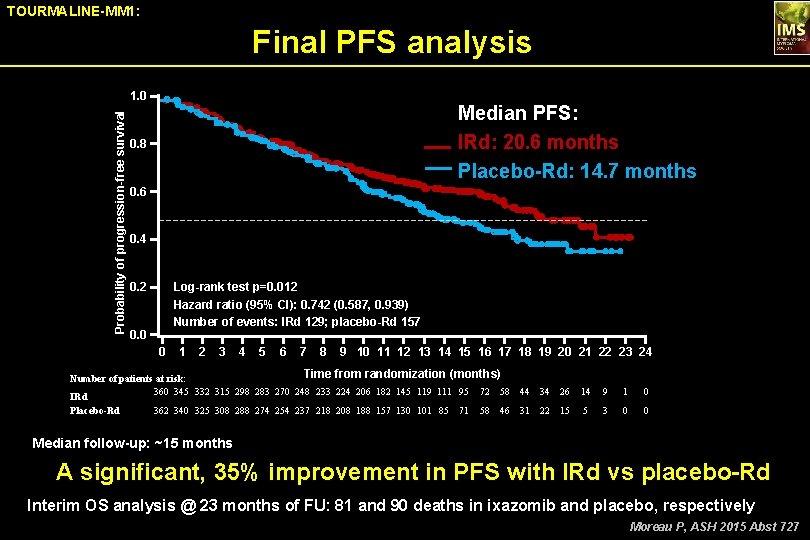
TOURMALINE-MM 1: Final PFS analysis Probability of progression-free survival 1. 0 Median PFS: IRd: 20. 6 months Placebo-Rd: 14. 7 months 0. 8 0. 6 0. 4 0. 2 Log-rank test p=0. 012 Hazard ratio (95% CI): 0. 742 (0. 587, 0. 939) Number of events: IRd 129; placebo-Rd 157 0. 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 Time from randomization (months) Number of patients at risk: 360 345 332 315 298 283 270 248 233 224 206 182 145 119 111 95 72 58 IRd Placebo-Rd 362 340 325 308 288 274 254 237 218 208 188 157 130 101 85 71 58 46 44 34 26 14 9 1 0 31 22 15 5 3 0 0 Median follow-up: ~15 months A significant, 35% improvement in PFS with IRd vs placebo-Rd Interim OS analysis @ 23 months of FU: 81 and 90 deaths in ixazomib and placebo, respectively Moreau P, ASH 2015 Abst 727

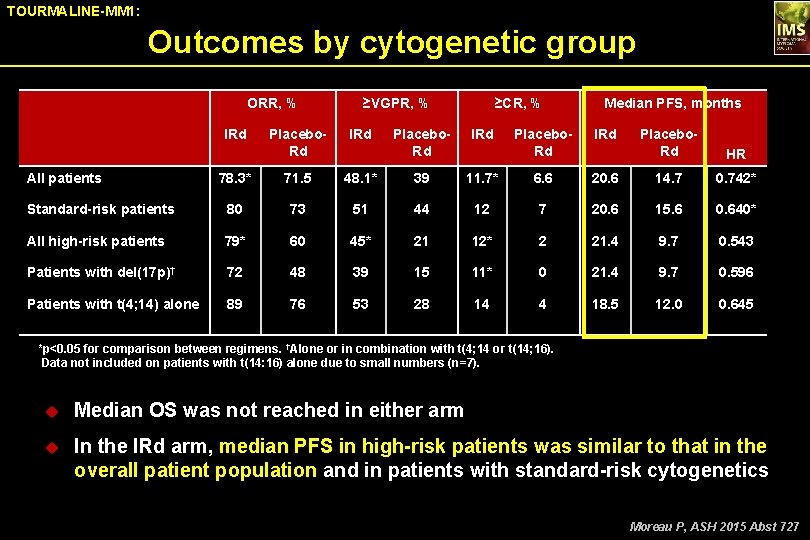
TOURMALINE-MM 1: Outcomes by cytogenetic group ORR, % IRd ≥VGPR, % Placebo. Rd IRd 78. 3* 71. 5 Standard-risk patients 80 All high-risk patients ≥CR, % Placebo. Rd IRd 48. 1* 39 73 51 79* 60 Patients with del(17 p)† 72 Patients with t(4; 14) alone 89 All patients Median PFS, months Placebo. Rd IRd Placebo. Rd HR 11. 7* 6. 6 20. 6 14. 7 0. 742* 44 12 7 20. 6 15. 6 0. 640* 45* 21 12* 2 21. 4 9. 7 0. 543 48 39 15 11* 0 21. 4 9. 7 0. 596 76 53 28 14 4 18. 5 12. 0 0. 645 *p<0. 05 for comparison between regimens. †Alone or in combination with t(4; 14 or t(14; 16). Data not included on patients with t(14: 16) alone due to small numbers (n=7). u Median OS was not reached in either arm u In the IRd arm, median PFS in high-risk patients was similar to that in the overall patient population and in patients with standard-risk cytogenetics Moreau P, ASH 2015 Abst 727

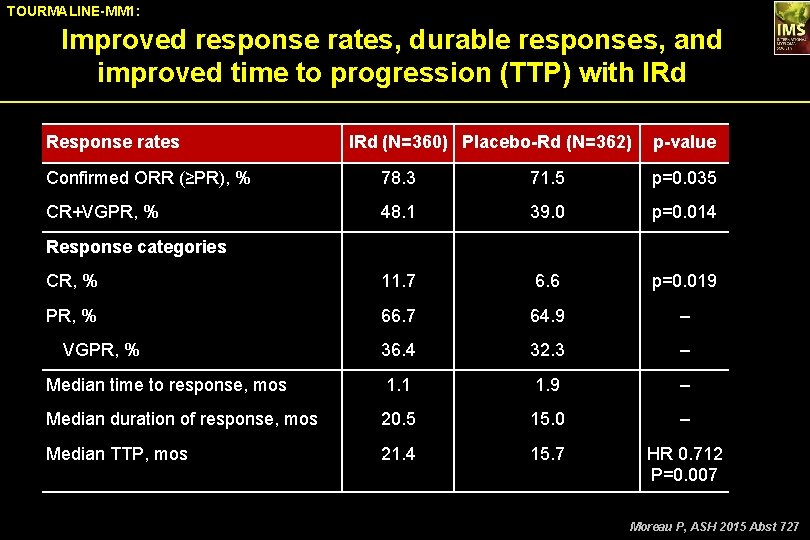
TOURMALINE-MM 1: Improved response rates, durable responses, and improved time to progression (TTP) with IRd Response rates IRd (N=360) Placebo-Rd (N=362) p-value Confirmed ORR (≥PR), % 78. 3 71. 5 p=0. 035 CR+VGPR, % 48. 1 39. 0 p=0. 014 CR, % 11. 7 6. 6 p=0. 019 PR, % 66. 7 64. 9 – 36. 4 32. 3 – Median time to response, mos 1. 1 1. 9 – Median duration of response, mos 20. 5 15. 0 – Median TTP, mos 21. 4 15. 7 HR 0. 712 P=0. 007 Response categories VGPR, % Moreau P, ASH 2015 Abst 727

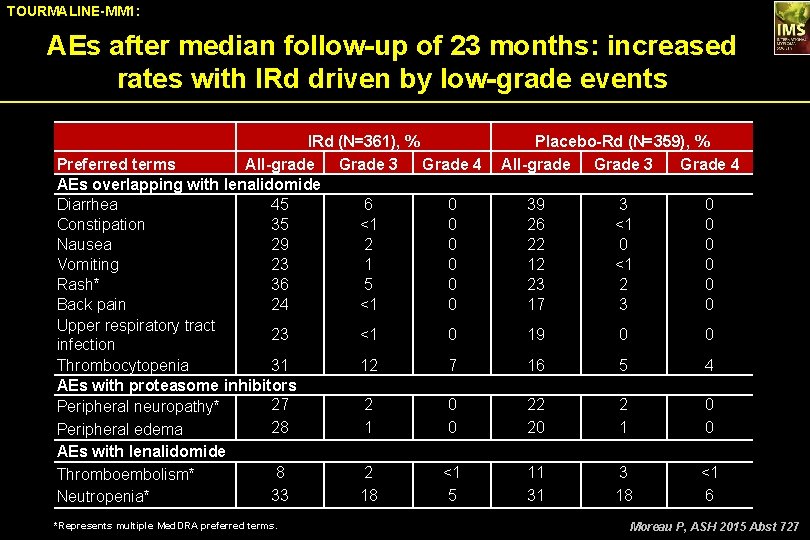
TOURMALINE-MM 1: AEs after median follow-up of 23 months: increased rates with IRd driven by low-grade events IRd (N=361), % Preferred terms All-grade Grade 3 Grade 4 AEs overlapping with lenalidomide Diarrhea 45 6 0 Constipation 35 <1 0 Nausea 29 2 0 Vomiting 23 1 0 Rash* 36 5 0 Back pain 24 <1 0 Upper respiratory tract 23 <1 0 infection Thrombocytopenia 31 12 7 AEs with proteasome inhibitors 27 2 0 Peripheral neuropathy* 28 1 0 Peripheral edema AEs with lenalidomide Thromboembolism* Neutropenia* 8 33 *Represents multiple Med. DRA preferred terms. 2 18 <1 5 Placebo-Rd (N=359), % All-grade Grade 3 Grade 4 39 26 22 12 23 17 3 <1 0 <1 2 3 0 0 0 19 0 0 16 5 4 22 20 2 1 0 0 11 31 3 18 <1 6 Moreau P, ASH 2015 Abst 727

TOURMALINE-MM 1: Conclusions • Ixazomib when combined with Rd for patients with relapsed and/or refractory MM was associated with: – a significant and clinically meaningful improvement in PFS – significantly improved TTP and response rates – improved PFS in high-risk patients, similar to that in the overall patient population and in standard-risk patients • Ixazomib added limited additional toxicity to that seen with placebo-Rd – Low rates of PN and no cardiovascular or renal signals – Patient-reported quality of life was maintained • The all-oral regimen of IRd may become a new standard of care for relapsed and/or refractory MM • Ixazomib was approved by the US FDA on Nov 20 under the name NINLARO ® Ixazomib, the first oral proteasome inhibitor, significantly extends PFS in a phase 3 trial Moreau P, ASH 2015 Abst 727

Weekly Carfilzomib With Dexamethasone for Patients With Relapsed or Refractory Multiple Myeloma: Updated Results From the Phase 1/2 Study CHAMPION-1 (NCT 01677858) James Berenson, 1 Alan Cartmell, 2 Roger Lyons, 3 Wael Harb, 4 Dimitrios Tzachanis, 5 Richy Agajanian, 6 Ralph Boccia, 7 Morton Coleman, 8 Robert A. Moss, 9 Robert M. Rifkin, 10 Marco Schupp, 11 Sandra Dixon, 11 Ying Ou, 11 Janet Anderl, 11 Jesus Berdeja 12 1 Institute for Myeloma & Bone Cancer Research, Los Angeles, CA; 2 Comprehensive Blood and Cancer Center, Bakersfield, CA; 3 US Oncology Research and Cancer Care Centers of South Texas, San Antonio, TX; 4 Horizon Oncology Center, Lafayette, IN; 5 Cedars-Sinai Medical Center, Los Angeles, CA; 6 The Oncology Institute of Hope and Innovation, Downey, CA; 7 Center for Cancer and Blood Disorders, Bethesda, MD; 8 New. York-Presbyterian/Weill Cornell, New York, NY; 9 Robert A Moss MD Inc. , Fountain Valley, CA; 10 US Oncology Research and Rocky Mountain Cancer Centers, Denver, CO; 11 Onyx Pharmaceuticals, Inc. , an Amgen subsidiary, South San Francisco, CA; 12 Sarah Cannon Research Institute, Nashville, TN Berenson J, ASH 2015 Abst 373

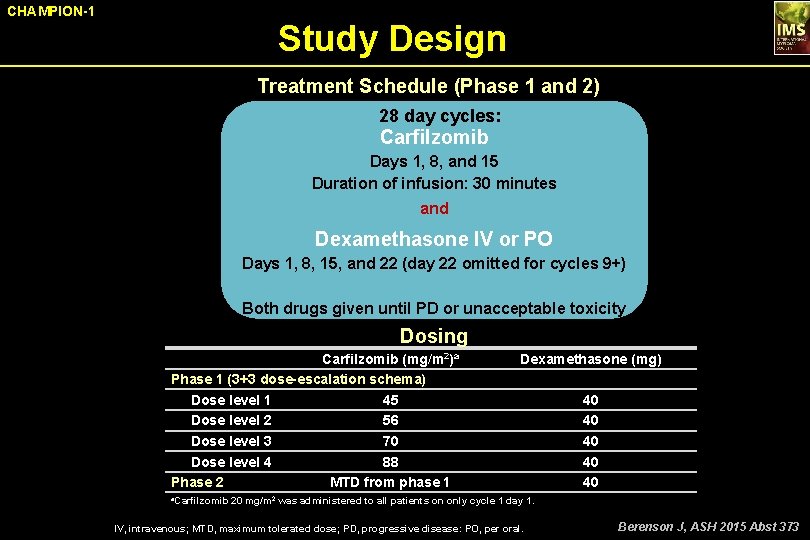
CHAMPION-1 Study Design Treatment Schedule (Phase 1 and 2) 28 day cycles: Carfilzomib Days 1, 8, and 15 Duration of infusion: 30 minutes and Dexamethasone IV or PO Days 1, 8, 15, and 22 (day 22 omitted for cycles 9+) Both drugs given until PD or unacceptable toxicity Dosing Carfilzomib (mg/m 2)a Phase 1 (3+3 dose-escalation schema) Dose level 1 45 Dose level 2 56 Dose level 3 70 Dose level 4 88 Phase 2 MTD from phase 1 Dexamethasone (mg) 40 40 40 a. Carfilzomib 20 mg/m 2 was administered to all patients on only cycle 1 day 1. IV, intravenous; MTD, maximum tolerated dose; PD, progressive disease: PO, per oral. Berenson J, ASH 2015 Abst 373

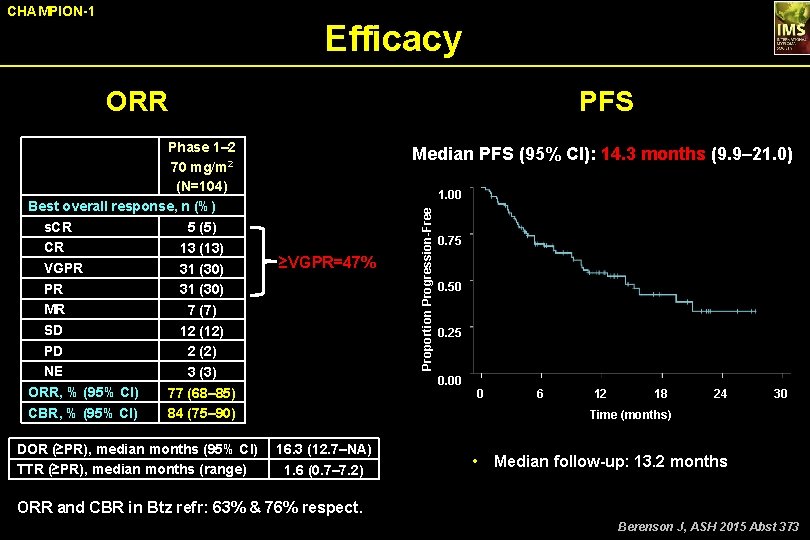
CHAMPION-1 Efficacy ORR DOR (≥PR), median months (95% CI) TTR (≥PR), median months (range) Median PFS (95% CI): 14. 3 months (9. 9– 21. 0) 1. 00 ≥VGPR=47% Proportion Progression-Free Phase 1– 2 70 mg/m 2 (N=104) Best overall response, n (%) s. CR 5 (5) CR 13 (13) VGPR 31 (30) MR 7 (7) SD 12 (12) PD 2 (2) NE 3 (3) ORR, % (95% CI) 77 (68– 85) CBR, % (95% CI) 84 (75– 90) PFS 0. 75 0. 50 0. 25 0. 00 0 6 12 18 24 30 Time (months) 16. 3 (12. 7–NA) 1. 6 (0. 7– 7. 2) • Median follow-up: 13. 2 months ORR and CBR in Btz refr: 63% & 76% respect. Berenson J, ASH 2015 Abst 373

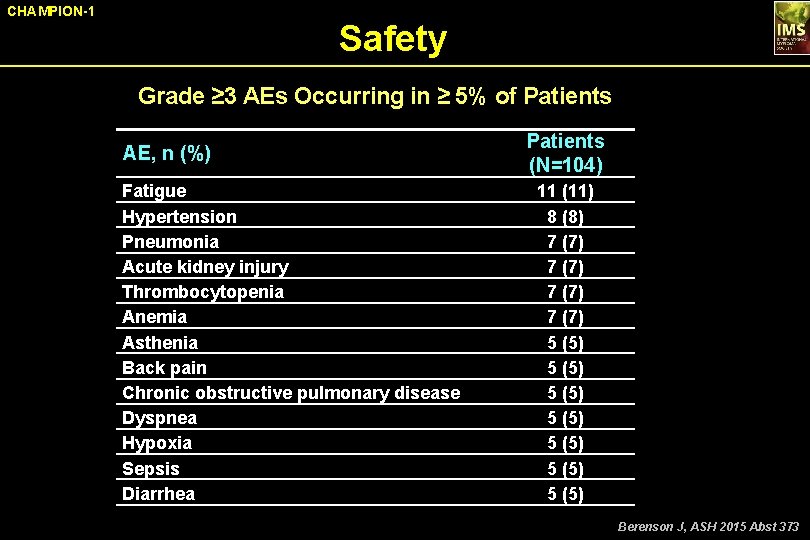
CHAMPION-1 Safety Grade ≥ 3 AEs Occurring in ≥ 5% of Patients AE, n (%) Fatigue Hypertension Pneumonia Acute kidney injury Thrombocytopenia Anemia Asthenia Back pain Chronic obstructive pulmonary disease Dyspnea Hypoxia Sepsis Diarrhea Patients (N=104) 11 (11) 8 (8) 7 (7) 5 (5) 5 (5) Berenson J, ASH 2015 Abst 373

CHAMPION-1 Conclusions I CHAMPION-1 study is the first clinical trial to investigate once weekly dosing of carfilzomib (administered on d 1, 8 and 15 of 28 day cycles) ‒ combined with once-weekly dexamethasone MTD of carfilzomib: 20/70 mg/m 2 (30 -min IV infusion) ‒ The incidence rates for specific grade ≥ 3 AEs of interest were similar to, or lower than, those observed with twice-weekly carfilzomib 1– 3 • Grade ≥ 3 cardiac failure (2%) occurred at a lower frequency than reported in phase 3 studies of twice‑weekly carfilzomib combination regimens 3, 4 ‒ Incidence rates of dose reductions/treatment discontinuations and deaths due to AEs were similar to those observed in ENDEAVOR: 4 • Dose reductions due to AEs: 23% Kd, 48% Vd; 17% CHAMPION-1 • Treatment discontinuation due to AEs: 14% Kd, 16% Vd; 13% CHAMPION-1 • On-study deaths due to AEs: 4% Kd, 3% Vd; 4% CHAMPION-1 Berenson J, ASH 2015 Abst 373

CHAMPION-1 Conclusions II At the MTD, the 77% ORR (84% CBR) in RRMM pts compares favorably ‒ Single-agent, once-weekly bortezomib (ORR of 55%)1 ‒ Twice-weekly carfilzomib at higher doses w/ dexamethasone for RRMM pts • Phase 1/2 trial at 20/45 or 20/56 mg/m 2 (ORR of 55%)2 • ENDEAVOR 3 at 20/56 mg/m 2 (ORR of 77% in which only 3% were refractory to bortezomib compared w/ 52% in CHAMPION-1 trial) The ORR was 63% for patients refractory to bortezomib ‒ Further supporting carfilzomib is effective for pts refractory to bortezomib 4 PK/PDn results support the efficacy of once-weekly, 70 mg/m 2 regimen Currently being examined in an ongoing, phase 3 superiority study ARROW (NCT 02412878) ‒ Once-weekly 20/70 mg/m 2 carfilzomib regimen vs twice-weekly 20/27 mg/m 2 carfilzomib for pts with RRMM Berenson J, ASH 2015 Abst 373

Pembrolizumab in Combination With Lenalidomide and Low-Dose Dexamethasone for Relapsed/Refractory Multiple Myeloma (RRMM): KEYNOTE-023 Jesus San Miguel, 1 Maria-Victoria Mateos, 2 Jatin J. Shah, 3 Enrique M. Ocio, 2 Paula Rodríguez-Otero, 1 Donna Reece, 4 Nihkil Munshi, 5 David Avigan, 6 Yang Ge, 7 Arun Balakumaran, 7 Patricia Marinello, 7 Robert Orlowski*, 3 David Siegel*8 1 Clinica Universidad de Navarra, Pamplona, Spain; 2 Complejo Asistencial Universitario de Salamanca/IBSAL, Salamanca, Spain; 3 The University of Texas MD Anderson Cancer Center, Houston, TX, USA; 4 Princess Margaret Cancer Centre, Toronto, ON, Canada; 5 Dana-Farber Cancer Institute, Boston, MA; 6 Beth Israel Deaconess Medical Center/Harvard Medical School, Boston, MA, USA; 7 Merck & Co, Inc, Kenilworth, NJ, USA; 8 Hackensack University Medical Center, Hackensack, NJ, USA San Miguel JF, ASH 2015 Abst 505

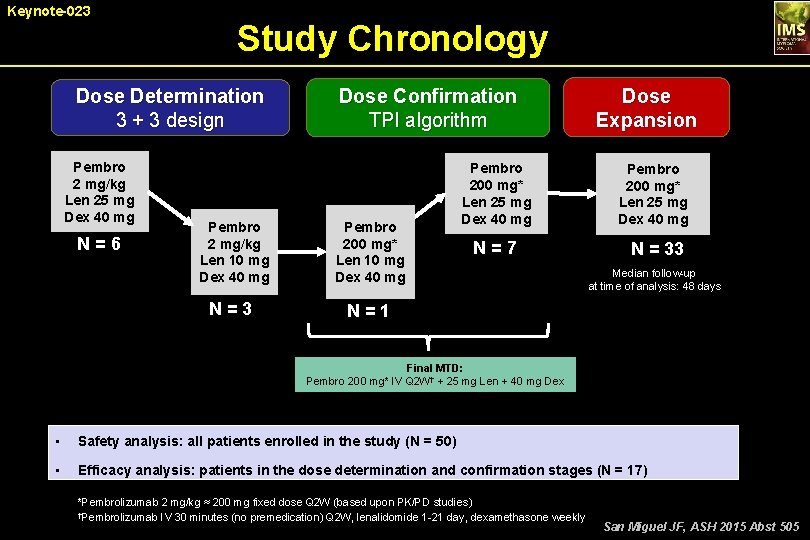
Keynote-023 Study Chronology Dose Determination 3 + 3 design Pembro 2 mg/kg Len 25 mg Dex 40 mg N = 6 Dose Confirmation TPI algorithm Pembro 2 mg/kg Len 10 mg Dex 40 mg Pembro 200 mg* Len 10 mg Dex 40 mg N = 3 N = 1 Pembro 200 mg* Len 25 mg Dex 40 mg N = 7 Dose Expansion Pembro 200 mg* Len 25 mg Dex 40 mg N = 33 Median follow-up at time of analysis: 48 days Final MTD: Pembro 200 mg* IV Q 2 W† + 25 mg Len + 40 mg Dex • Safety analysis: all patients enrolled in the study (N = 50) • Efficacy analysis: patients in the dose determination and confirmation stages (N = 17) *Pembrolizumab 2 mg/kg ≈ 200 mg fixed dose Q 2 W (based upon PK/PD studies) †Pembrolizumab IV 30 minutes (no premedication) Q 2 W, lenalidomide 1 -21 day, dexamethasone weekly San Miguel JF, ASH 2015 Abst 505

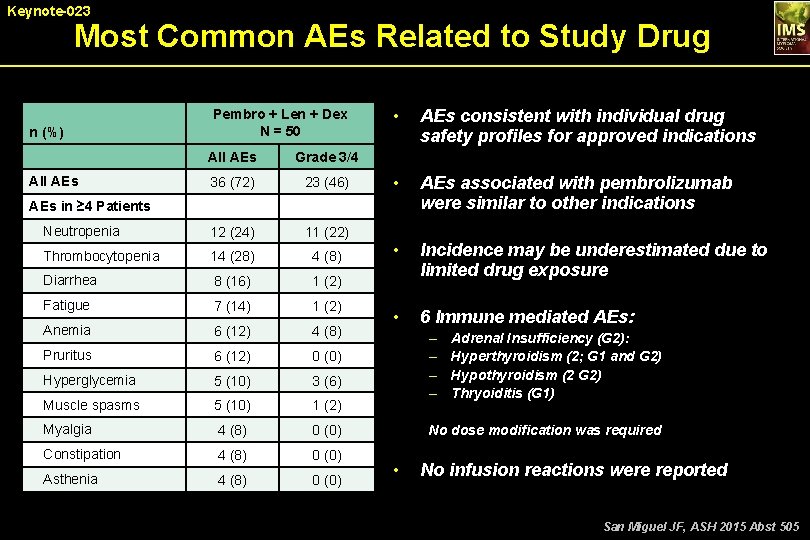
Keynote-023 Most Common AEs Related to Study Drug n (%) Pembro + Len + Dex N = 50 All AEs Grade 3/4 36 (72) 23 (46) Neutropenia 12 (24) 11 (22) Thrombocytopenia 14 (28) 4 (8) Diarrhea 8 (16) 1 (2) Fatigue 7 (14) 1 (2) Anemia 6 (12) 4 (8) Pruritus 6 (12) 0 (0) Hyperglycemia 5 (10) 3 (6) Muscle spasms 5 (10) 1 (2) Myalgia 4 (8) 0 (0) Constipation 4 (8) 0 (0) Asthenia 4 (8) 0 (0) All AEs • AEs consistent with individual drug safety profiles for approved indications • AEs associated with pembrolizumab were similar to other indications • Incidence may be underestimated due to limited drug exposure • 6 Immune mediated AEs: AEs in ≥ 4 Patients – – Adrenal Insufficiency (G 2): Hyperthyroidism (2; G 1 and G 2) Hypothyroidism (2 G 2) Thryoiditis (G 1) No dose modification was required • No infusion reactions were reported San Miguel JF, ASH 2015 Abst 505

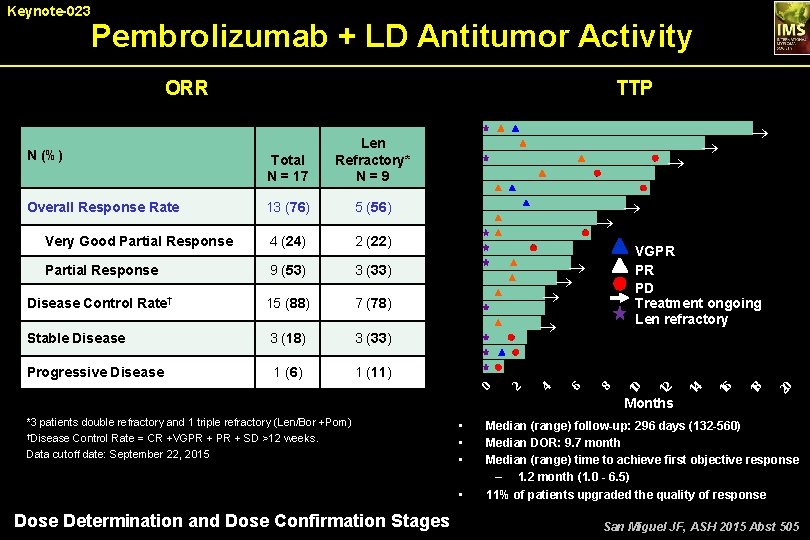
Pembrolizumab + LD Antitumor Activity TTP ORR 3 (33) Disease Control Rate† 15 (88) 7 (78) Stable Disease 3 (18) 3 (33) Progressive Disease 1 (6) 1 (11) ® ® 20 9 (53) 18 Partial Response VGPR PR PD Treatment ongoing Len refractory 16 2 (22) 14 4 (24) ® ® ® 12 Very Good Partial Response ® 8 5 (56) 6 13 (76) 4 Overall Response Rate ® 2 Total N = 17 0 N (%) Len Refractory* N = 9 10 Keynote-023 Months *3 patients double refractory and 1 triple refractory (Len/Bor +Pom) †Disease Control Rate = CR +VGPR + SD >12 weeks. Data cutoff date: September 22, 2015 • • Dose Determination and Dose Confirmation Stages Median (range) follow-up: 296 days (132 -560) Median DOR: 9. 7 month Median (range) time to achieve first objective response – 1. 2 month (1. 0 - 6. 5) 11% of patients upgraded the quality of response San Miguel JF, ASH 2015 Abst 505

Keynote-023 Conclusions • MTD/MAD was defined as pembrolizumab 200 mg in combination with lenalidomide 25 mg and low-dose dexamethasone 40 mg • Preliminary data suggest that this treatment combination has an acceptable safety and tolerability profile, and is consistent with AEs reported for pembrolizumab in solid tumors • Initial efficacy results show promising activity in heavily pretreated patients with RRMM and support the continued development of pembrolizumab in patients with multiple myeloma San Miguel JF, ASH 2015 Abst 505

A Phase II Study of Pembrolizumab, Pomalidomide and Dexamethasone in Patients with Relapsed/Refractory Multiple Myeloma Ashraf Badros, Mehmet Kocoglu, Ning Ma, Aaron Rapoport, Emily Lederer, Sunita Philip, Patricia Lesho, Cameron Dell, Nancy Hardy, Jean Yared, Olga Goloubeva and Zeba Singh University of Maryland, Baltimore MD Investigator initiated study supported partially by Merck Clinical. Trials. gov # NCT 02289222 Badros A, ASH 2015 Abst 506

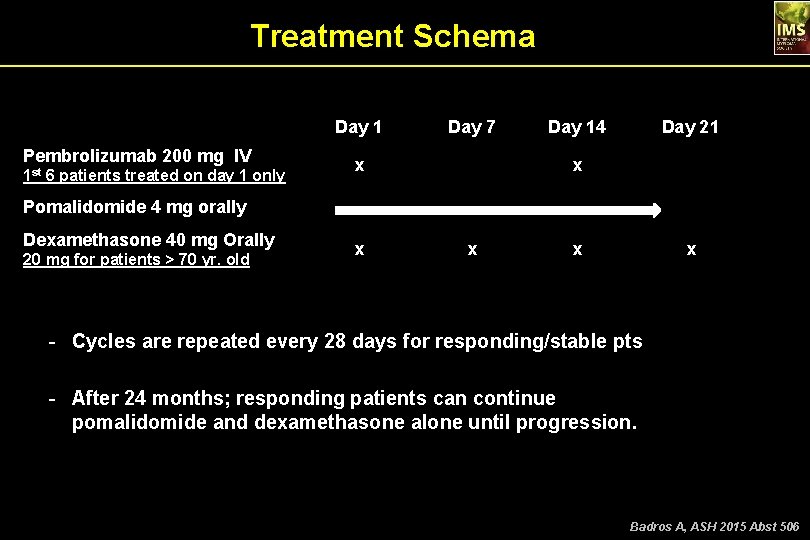
Treatment Schema Day 1 Pembrolizumab 200 mg IV x 1 st 6 patients treated on day 1 only Pomalidomide 4 mg orally Dexamethasone 40 mg Orally 20 mg for patients > 70 yr. old Day 7 Day 14 Day 21 x x x - Cycles are repeated every 28 days for responding/stable pts - After 24 months; responding patients can continue pomalidomide and dexamethasone alone until progression. Badros A, ASH 2015 Abst 506

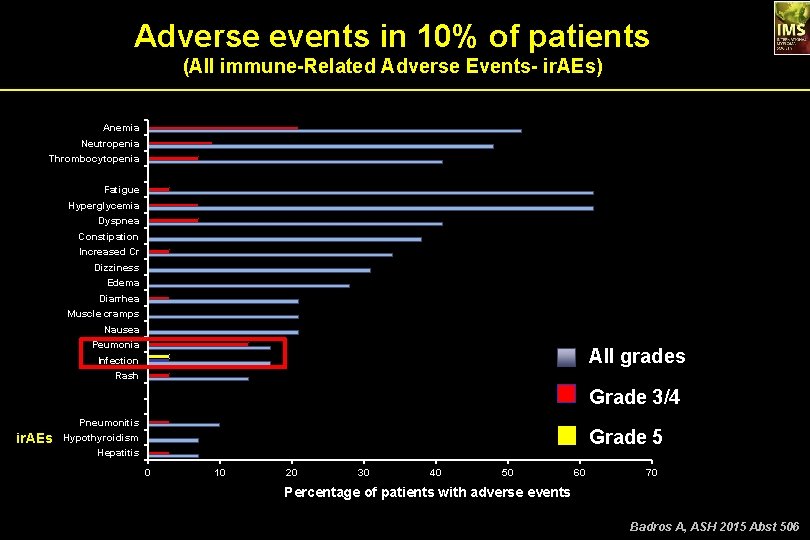
Adverse events in 10% of patients (All immune-Related Adverse Events- ir. AEs) Anemia Neutropenia Thrombocytopenia Fatigue Hyperglycemia Dyspnea Constipation Increased Cr Dizziness Edema Diarrhea Muscle cramps Nausea Peumonia All grades Infection Rash Grade 3/4 Pneumonitis ir. AEs Grade 5 Hypothyroidism Hepatitis 0 10 20 30 40 50 60 70 Percentage of patients with adverse events Badros A, ASH 2015 Abst 506

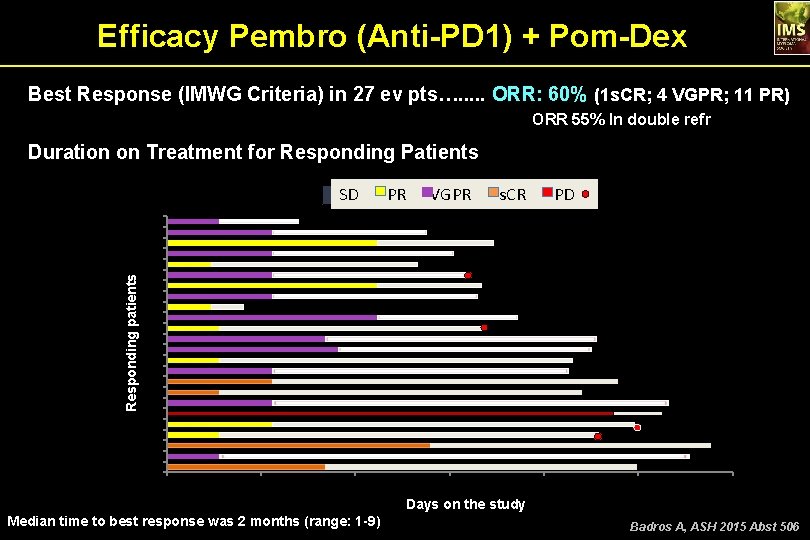
Efficacy Pembro (Anti-PD 1) + Pom-Dex Best Response (IMWG Criteria) in 27 ev pts…. . . ORR: 60% (1 s. CR; 4 VGPR; 11 PR) ORR 55% In double refr Duration on Treatment for Responding Patients Responding patients SD PR VGPR s. CR SD PR VGPR s. CR PD 24 23 22 21 20 19 18 17 16 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 0 50 100 150 200 250 300 Days on the study Median time to best response was 2 months (range: 1 -9) Badros A, ASH 2015 Abst 506

Daratumumab in Combination With Lenalidomide and Dexamethasone in Patients With Relapsed or Relapsed and Refractory Multiple Myeloma: Updated Results of a Phase 1/2 Study (GEN 503) • • Torben Plesner, MD, Ph. D 1; Hendrik-Tobias Arkenau, MD 2; Peter Gimsing, MD, Ph. D 3; Jakub Krejcik, MD 1; Charlotte Lemech, MD 2; Monique C. Minnema, MD, Ph. D 4; Ulrik Lassen, MD, Ph. D 3; Jacob P. Laubach, MD 5; Antonio Palumbo, MD 6; Steen Lisby, MD 7; Linda Basse, MD, DMSc 7; Jianping Wang, Ph. D 8; Kate Sasser, Ph. D 9; Mary E. Guckert, MSN, RN 9; Howard Yeh, MD 8; Tahamtan Ahmadi, MD, Ph. D 9; Henk M. Lokhorst, MD, Ph. D 10; Paul G. Richardson, MD 5 1 Vejle Hospital, Vejle, Denmark; 2 Sarah Cannon Research Institute, London, UK; 3 Department of Haematology, Rigshospitalet, University of Copenhagen, Denmark; 4 Department of Hematology, UMC Utrecht Cancer Center, Utrecht, The Netherlands; 5 The Le. Bow Institute for Myeloma Therapeutics and the Jerome Lipper Myeloma Center, Department of Medical Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, USA; 6 Myeloma Unit, Division of Hematology, University of Torino, Italy; 7 Genmab A/S, Copenhagen, Denmark; 8 Janssen Research & Development, LLC, Raritan, NJ, USA; 9 Janssen Research & Development, LLC, Spring House, PA, USA; 10 Department of Hematology, VU University Medical Center, Amsterdam, The Netherlands. Plesner T, ASH 2015 Abst 507

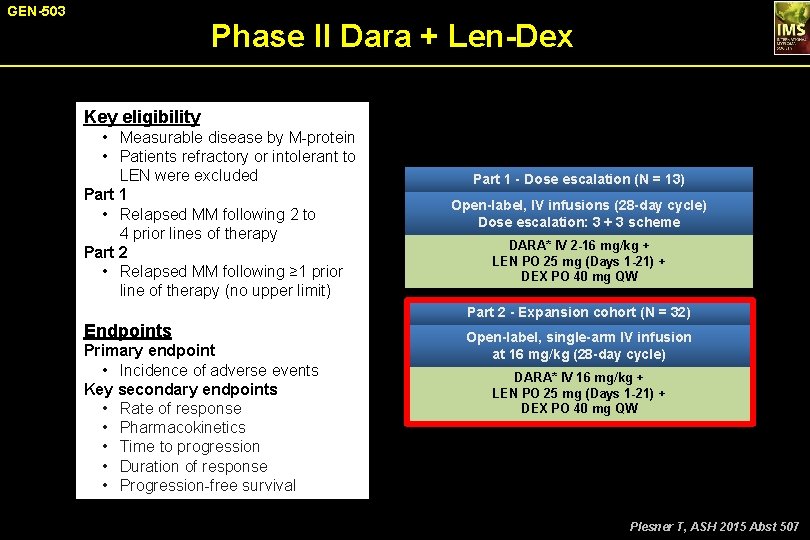
GEN-503 Phase II Dara + Len-Dex Key eligibility • Measurable disease by M-protein • Patients refractory or intolerant to LEN were excluded Part 1 • Relapsed MM following 2 to 4 prior lines of therapy Part 2 • Relapsed MM following ≥ 1 prior line of therapy (no upper limit) Part 1 - Dose escalation (N = 13) Open-label, IV infusions (28 -day cycle) Dose escalation: 3 + 3 scheme DARA* IV 2 -16 mg/kg + LEN PO 25 mg (Days 1 -21) + DEX PO 40 mg QW Part 2 - Expansion cohort (N = 32) Endpoints Primary endpoint • Incidence of adverse events Key secondary endpoints • Rate of response • Pharmacokinetics • Time to progression • Duration of response • Progression-free survival Open-label, single-arm IV infusion at 16 mg/kg (28 -day cycle) DARA* IV 16 mg/kg + LEN PO 25 mg (Days 1 -21) + DEX PO 40 mg QW *QW for Months 1 -2, Q 2 W for Months 3 -6, and Q 4 W beyond. Plesner T, ASH 2015 Abst 507

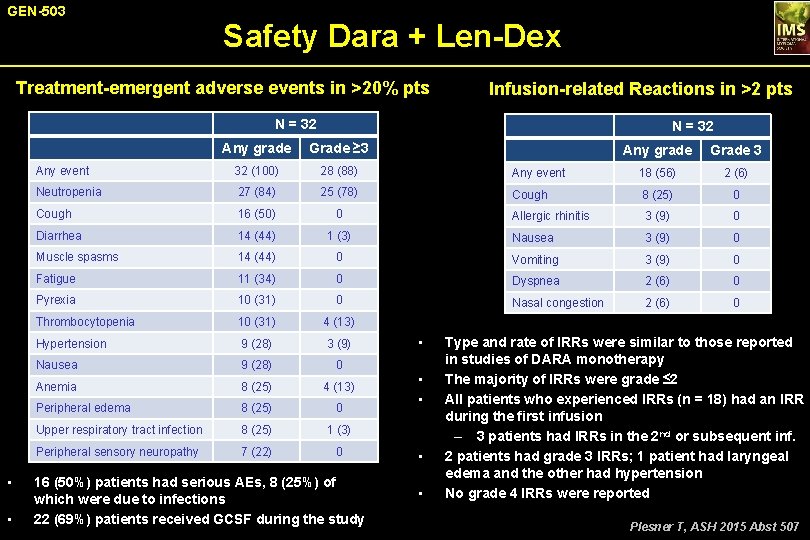
GEN-503 Safety Dara + Len-Dex Treatment-emergent adverse events in >20% pts Infusion-related Reactions in >2 pts N = 32 • • N = 32 Any grade Grade ≥ 3 Any event 32 (100) 28 (88) Neutropenia 27 (84) 25 (78) Cough 16 (50) 0 Diarrhea 14 (44) Muscle spasms Any grade Grade 3 Any event 18 (56) 2 (6) Cough 8 (25) 0 Allergic rhinitis 3 (9) 0 1 (3) Nausea 3 (9) 0 14 (44) 0 Vomiting 3 (9) 0 Fatigue 11 (34) 0 Dyspnea 2 (6) 0 Pyrexia 10 (31) 0 Nasal congestion 2 (6) 0 Thrombocytopenia 10 (31) 4 (13) Hypertension 9 (28) 3 (9) Nausea 9 (28) 0 Anemia 8 (25) 4 (13) Peripheral edema 8 (25) 0 Upper respiratory tract infection 8 (25) 1 (3) Peripheral sensory neuropathy 7 (22) 0 16 (50%) patients had serious AEs, 8 (25%) of which were due to infections 22 (69%) patients received GCSF during the study • • • Type and rate of IRRs were similar to those reported in studies of DARA monotherapy The majority of IRRs were grade ≤ 2 All patients who experienced IRRs (n = 18) had an IRR during the first infusion – 3 patients had IRRs in the 2 nd or subsequent inf. 2 patients had grade 3 IRRs; 1 patient had laryngeal edema and the other had hypertension No grade 4 IRRs were reported Plesner T, ASH 2015 Abst 507

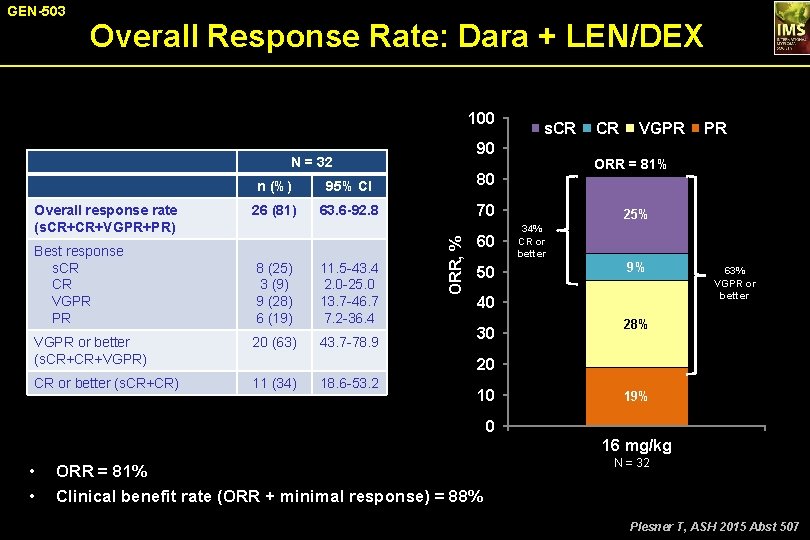
GEN-503 Overall Response Rate: Dara + LEN/DEX 100 90 N = 32 Best response s. CR CR VGPR PR n (%) 95% CI 80 26 (81) 63. 6 -92. 8 70 8 (25) 3 (9) 9 (28) 6 (19) 11. 5 -43. 4 2. 0 -25. 0 13. 7 -46. 7 7. 2 -36. 4 VGPR or better (s. CR+CR+VGPR) 20 (63) 43. 7 -78. 9 CR or better (s. CR+CR) 11 (34) ORR, % Overall response rate (s. CR+CR+VGPR+PR) s. CR 60 50 CR VGPR ORR = 81% 25% 34% CR or better 9% 40 30 PR 63% VGPR or better 28% 20 18. 6 -53. 2 10 19% 0 16 mg/kg • • ORR = 81% Clinical benefit rate (ORR + minimal response) = 88% N = 32 Plesner T, ASH 2015 Abst 507

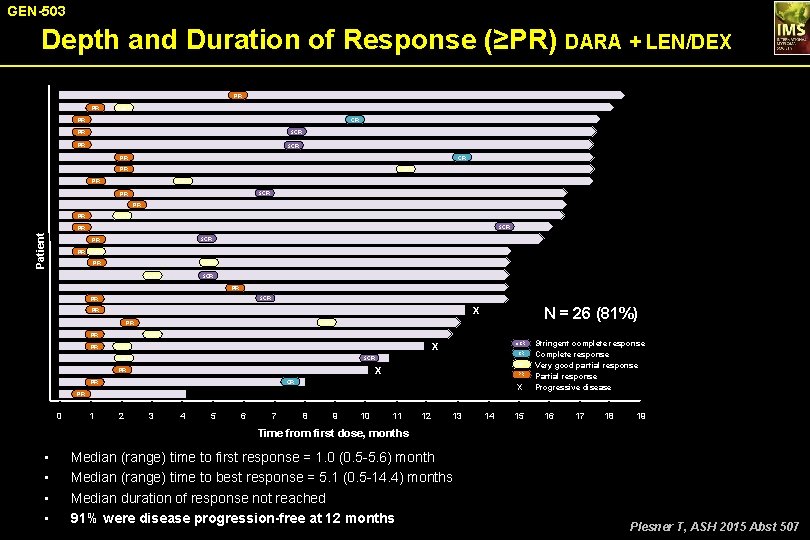
GEN-503 Depth and Duration of Response (≥PR) DARA + LEN/DEX PR PR VGPR PR CR s. CR PR PR s. CR PR VGPR PR s. CR PR PR PR VGPR s. CR Patient PR s. CR PR PR VGPR s. CR PR X PR PR PR N = 26 (81%) VGPR PR VGPR Stringent complete response CR Complete response VGPR Very good partial response PR Partial response X Progressive disease X VGPR s. CR X PR CR PR PR 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 Time from first dose, months • • Median (range) time to first response = 1. 0 (0. 5 -5. 6) month Median (range) time to best response = 5. 1 (0. 5 -14. 4) months Median duration of response not reached 91% were disease progression-free at 12 months Plesner T, ASH 2015 Abst 507

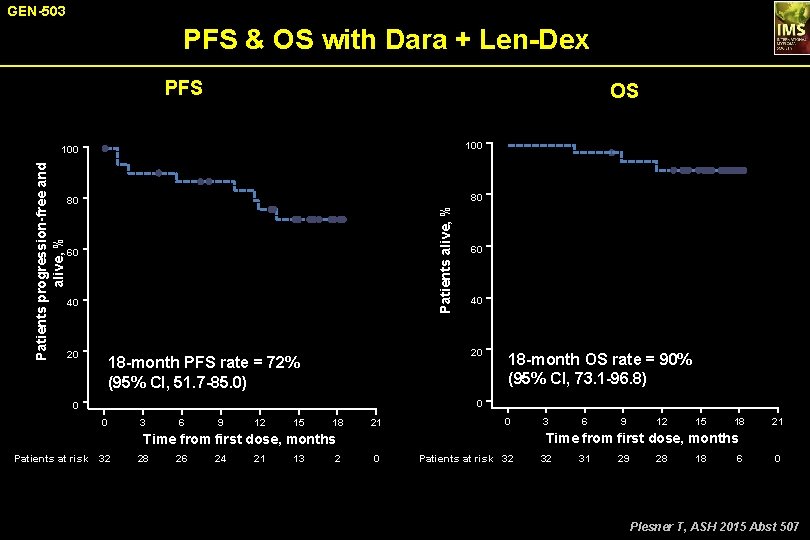
GEN-503 PFS & OS with Dara + Len-Dex PFS OS 100 80 80 Patients alive, % Patients progression-free and alive, % 100 60 40 20 18 -month PFS rate = 72% (95% CI, 51. 7 -85. 0) 18 -month OS rate = 90% (95% CI, 73. 1 -96. 8) 0 0 0 3 6 9 12 15 18 21 0 28 26 24 21 13 2 6 9 12 15 18 21 Time from first dose, months Patients at risk 32 3 0 Patients at risk 32 32 31 29 28 18 6 0 Plesner T, ASH 2015 Abst 507

GEN-503 Conclusions • DARA + LEN/DEX induced rapid, deep, and durable responses – At a median follow-up time of 15. 6 months, ORR was 81% including 28% VGPR and 34% CR/s. CR – Median time to first response was 1 month – PFS rate of 72% at 18 months – OS rate of 90% at 18 months • DARA can be safely combined with LEN/DEX with no additional safety signals • Randomized phase 3 studies of DARA are ongoing: – DARA + LEN/DEX in relapsed/refractory patients (POLLUX)* – DARA + LEN/DEX in newly diagnosed patients (MAIA)† Plesner T, ASH 2015 Abst 507

Open-label, Multicenter, Phase 1 b Study of Daratumumab in Combination With Pomalidomide and Dexamethasone in Patients With ≥ 2 Lines of Prior Therapy and Refractory or Relapsed and Refractory Multiple Myeloma (MM) • Ajai Chari, MD 1; Sagar Lonial, MD 2; Attaya Suvannasankha, MD 3; Joseph W. Fay, MD 4; Bertrand Arnulf, MD, Ph. D 5; Jainulabdeen J. Ifthikharuddin, MD 6; Xiang Qin, MS 7; Tara Masterson, MS 7; Kerri Nottage, MD, MPH 8; Jordan Schecter, MD 8; Tahamtan Ahmadi, MD, Ph. D 7; Brendan Weiss, MD 9; Amrita Krishnan, MD 10; Suzanne Lentzsch, MD, Ph. D 11 • 1 Tisch Cancer Institute, Mount Sinai School of Medicine, New York, NY, USA; 2 Department of Hematology and Medical Oncology, Winship Cancer Institute, Emory University, Atlanta, GA, USA; 3 Indiana University School of Medicine and Simon Cancer Center, Richard L. Roudebush VAMC, Indianapolis, IN, USA; 4 Baylor Institute for Immunology Research, Dallas, TX, USA; 5 Hôpital Saint Louis, Paris, France; 6 James P. Wilmot Cancer Center, University of Rochester Strong Memorial Hospital, Rochester, NY, USA; 7 Janssen Research & Development, LLC, Spring House, PA, USA; 8 Janssen Research & Development, LLC, Raritan, NJ; 9 Abramson Cancer Center and Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA; 10 City of Hope, Duarte, CA, USA; 11 Columbia University Medical Center, New York, NY, USA. Chari A, ASH 2015 Abst 508

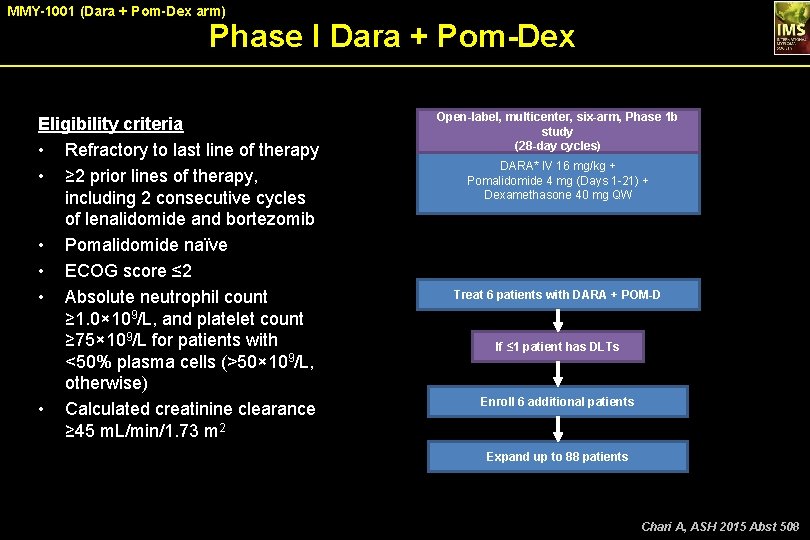
MMY-1001 (Dara + Pom-Dex arm) Phase I Dara + Pom-Dex Eligibility criteria • Refractory to last line of therapy • ≥ 2 prior lines of therapy, including 2 consecutive cycles of lenalidomide and bortezomib • Pomalidomide naïve • ECOG score ≤ 2 • Absolute neutrophil count ≥ 1. 0× 109/L, and platelet count ≥ 75× 109/L for patients with <50% plasma cells (>50× 109/L, otherwise) • Calculated creatinine clearance ≥ 45 m. L/min/1. 73 m 2 Open-label, multicenter, six-arm, Phase 1 b study (28 -day cycles) DARA* IV 16 mg/kg + Pomalidomide 4 mg (Days 1 -21) + Dexamethasone 40 mg QW *QW for Cycles 1 -2, Q 2 W for Cycles 3 -6, and Q 4 W beyond. Treat 6 patients with DARA + POM-D If ≤ 1 patient has DLTs Enroll 6 additional patients Expand up to 88 patients Chari A, ASH 2015 Abst 508

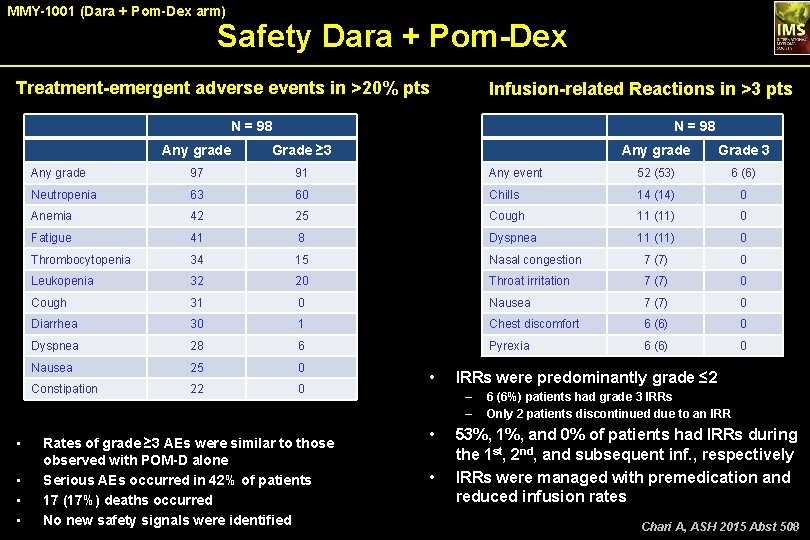
MMY-1001 (Dara + Pom-Dex arm) Safety Dara + Pom-Dex Treatment-emergent adverse events in >20% pts Infusion-related Reactions in >3 pts N = 98 • • N = 98 Any grade Grade ≥ 3 Any grade Grade 3 Any grade 97 91 Any event 52 (53) 6 (6) Neutropenia 63 60 Chills 14 (14) 0 Anemia 42 25 Cough 11 (11) 0 Fatigue 41 8 Dyspnea 11 (11) 0 Thrombocytopenia 34 15 Nasal congestion 7 (7) 0 Leukopenia 32 20 Throat irritation 7 (7) 0 Cough 31 0 Nausea 7 (7) 0 Diarrhea 30 1 Chest discomfort 6 (6) 0 Dyspnea 28 6 Pyrexia 6 (6) 0 Nausea 25 0 Constipation 22 0 Rates of grade ≥ 3 AEs were similar to those observed with POM-D alone Serious AEs occurred in 42% of patients 17 (17%) deaths occurred No new safety signals were identified • IRRs were predominantly grade ≤ 2 – – • • 6 (6%) patients had grade 3 IRRs Only 2 patients discontinued due to an IRR 53%, 1%, and 0% of patients had IRRs during the 1 st, 2 nd, and subsequent inf. , respectively IRRs were managed with premedication and reduced infusion rates Chari A, ASH 2015 Abst 508

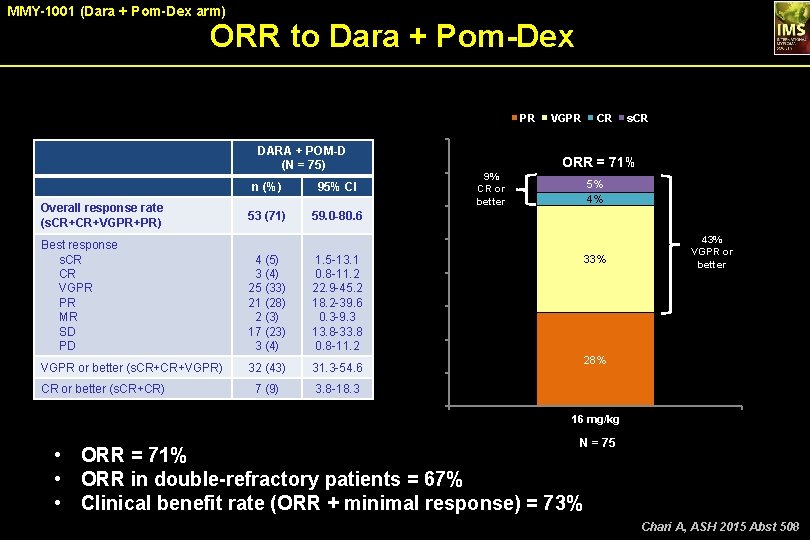
MMY-1001 (Dara + Pom-Dex arm) ORR to Dara + Pom-Dex PR 53 (71) 59. 0 -80. 6 60 9% CR or better 4 (5) 3 (4) 25 (33) 21 (28) 2 (3) 17 (23) 3 (4) 1. 5 -13. 1 0. 8 -11. 2 22. 9 -45. 2 18. 2 -39. 6 0. 3 -9. 3 13. 8 -33. 8 0. 8 -11. 2 VGPR or better (s. CR+CR+VGPR) 32 (43) 31. 3 -54. 6 7 (9) 3. 8 -18. 3 s. CR ORR = 71% 5% 4% 50 Best response s. CR CR VGPR PR MR SD PD CR or better (s. CR+CR) 70 ORR, % Overall response rate (s. CR+CR+VGPR+PR) 95% CI CR 80 DARA + POM-D (N = 75) n (%) VGPR 33% 40 43% VGPR or better 30 20 28% 10 0 16 mg/kg N = 75 • ORR = 71% • ORR in double-refractory patients = 67% • Clinical benefit rate (ORR + minimal response) = 73% Chari A, ASH 2015 Abst 508

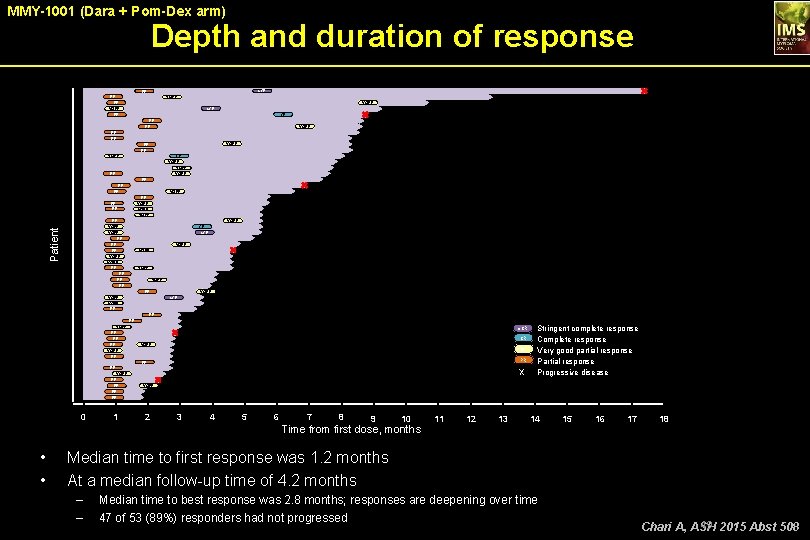
MMY-1001 (Dara + Pom-Dex arm) Depth and duration of response s. CR PR PR PR VGPR s. CR CR PR PR PR VGPR PR PR CR VGPR PR PR VGPR VGPR Patient PR VGPR PR PR CR s. CR VGPR PR PR VGPR PR VGPR s. CR PR PR VGPR PR Stringent complete response CR Complete response VGPR Very good partial response PR Partial response X Progressive disease s. CR VGPR PR VGPR PR 0 1 2 3 4 5 6 7 8 9 10 Time from first dose, months • • 11 12 13 14 15 16 17 18 Median time to first response was 1. 2 months At a median follow-up time of 4. 2 months – – Median time to best response was 2. 8 months; responses are deepening over time 47 of 53 (89%) responders had not progressed 49 2015 Abst 508 Chari A, ASH

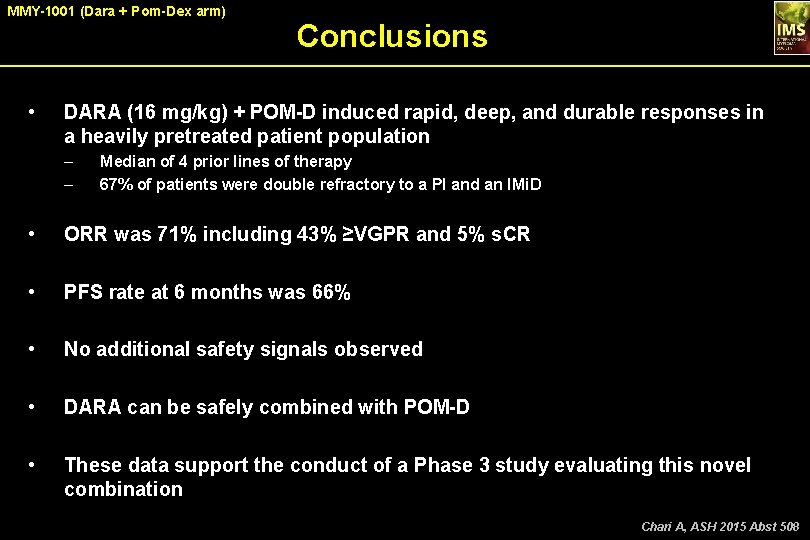
MMY-1001 (Dara + Pom-Dex arm) • Conclusions DARA (16 mg/kg) + POM-D induced rapid, deep, and durable responses in a heavily pretreated patient population – – Median of 4 prior lines of therapy 67% of patients were double refractory to a PI and an IMi. D • ORR was 71% including 43% ≥VGPR and 5% s. CR • PFS rate at 6 months was 66% • No additional safety signals observed • DARA can be safely combined with POM-D • These data support the conduct of a Phase 3 study evaluating this novel combination Chari A, ASH 2015 Abst 508

Clinical Efficacy of Daratumumab Monotherapy in Patients With Heavily Pretreated Relapsed or Refractory Multiple Myeloma Saad Z. Usmani, MD 1; Brendan M. Weiss, MD 2; Nizar J. Bahlis, MD 3; Andrew Belch, MD 4; Sagar Lonial, MD 5; Henk M. Lokhorst, MD 6; Peter M. Voorhees, MD 7; Paul G. Richardson, MD 8; A. Kate Sasser, Ph. D 9; Amy Axel Ph. D 9; Huaibao Feng, Ph. D 10; Clarissa M. Uhlar, Ph. D 9; Jianping Wang, Ph. D 9; Imran Khan, MD 10; Tahamtan Ahmadi, MD 9; Hareth Nahi, MD 1 Usmani S, ASH 2015 Abst 29

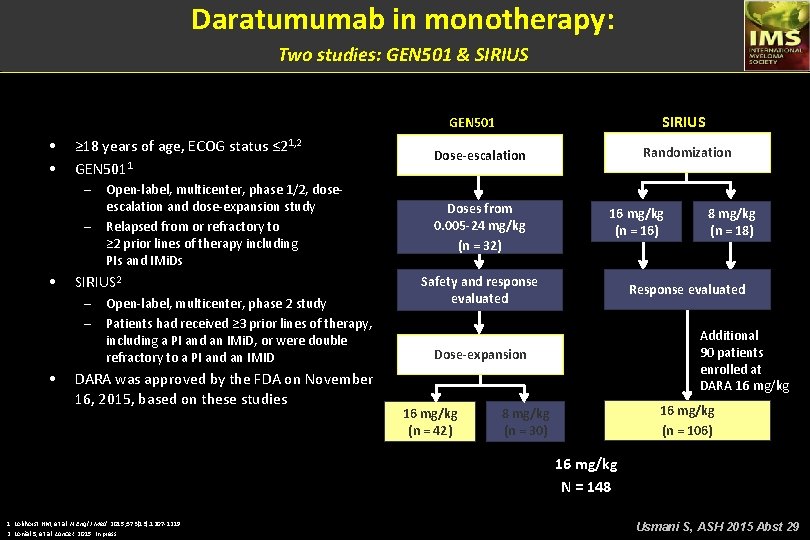
Daratumumab in monotherapy: Two studies: GEN 501 & SIRIUS GEN 501 • • ≥ 18 years of age, ECOG status ≤ 21, 2 GEN 5011 – – • SIRIUS 2 – – • Open-label, multicenter, phase 1/2, doseescalation and dose-expansion study Relapsed from or refractory to ≥ 2 prior lines of therapy including PIs and IMi. Ds Open-label, multicenter, phase 2 study Patients had received ≥ 3 prior lines of therapy, including a PI and an IMi. D, or were double refractory to a PI and an IMID DARA was approved by the FDA on November 16, 2015, based on these studies Randomization Dose-escalation Doses from 0. 005 -24 mg/kg (n = 32) 16 mg/kg (n = 16) Safety and response evaluated Response evaluated Additional 90 patients enrolled at DARA 16 mg/kg Dose-expansion 16 mg/kg (n = 42) 8 mg/kg (n = 18) 16 mg/kg (n = 106) 8 mg/kg (n = 30) 16 mg/kg N = 148 1. Lokhorst HM, et al. N Engl J Med. 2015; 373(13): 1207 -1219. 2. Lonial S, et al. Lancet. 2015. In press. Usmani S, ASH 2015 Abst 29

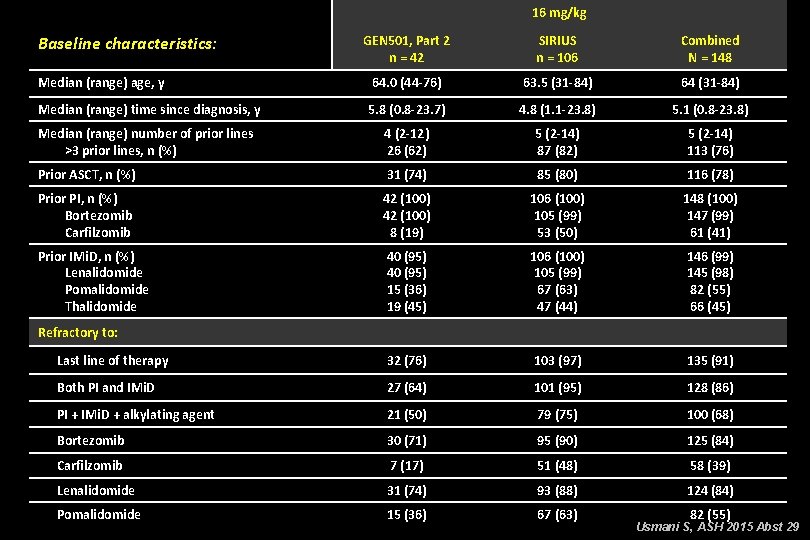
16 mg/kg Baseline characteristics: GEN 501, Part 2 n = 42 SIRIUS n = 106 Combined N = 148 Median (range) age, y 64. 0 (44 -76) 63. 5 (31 -84) 64 (31 -84) Median (range) time since diagnosis, y 5. 8 (0. 8 -23. 7) 4. 8 (1. 1 -23. 8) 5. 1 (0. 8 -23. 8) Median (range) number of prior lines >3 prior lines, n (%) 4 (2 -12) 26 (62) 5 (2 -14) 87 (82) 5 (2 -14) 113 (76) Prior ASCT, n (%) 31 (74) 85 (80) 116 (78) Prior PI, n (%) Bortezomib Carfilzomib 42 (100) 8 (19) 106 (100) 105 (99) 53 (50) 148 (100) 147 (99) 61 (41) Prior IMi. D, n (%) Lenalidomide Pomalidomide Thalidomide 40 (95) 15 (36) 19 (45) 106 (100) 105 (99) 67 (63) 47 (44) 146 (99) 145 (98) 82 (55) 66 (45) Last line of therapy 32 (76) 103 (97) 135 (91) Both PI and IMi. D 27 (64) 101 (95) 128 (86) PI + IMi. D + alkylating agent 21 (50) 79 (75) 100 (68) Bortezomib 30 (71) 95 (90) 125 (84) Carfilzomib 7 (17) 51 (48) 58 (39) Lenalidomide 31 (74) 93 (88) 124 (84) Pomalidomide 15 (36) 67 (63) Refractory to: 82 (55) Usmani S, ASH 2015 Abst 29

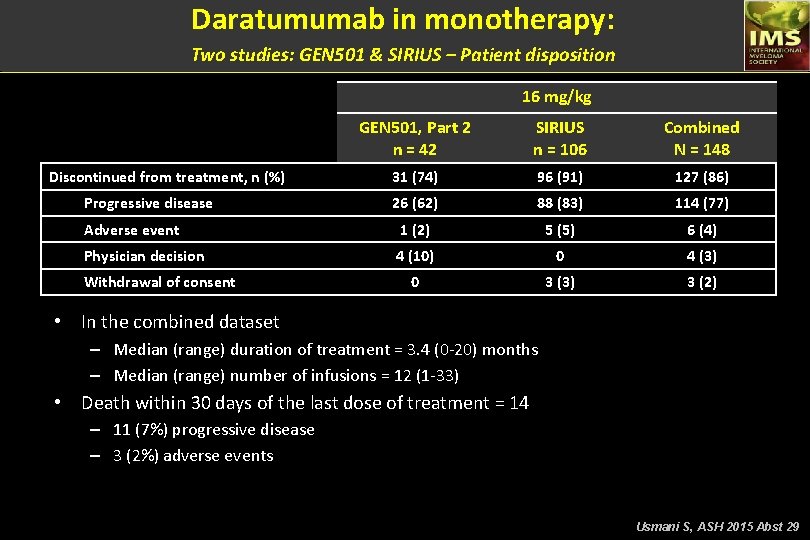
Daratumumab in monotherapy: Two studies: GEN 501 & SIRIUS – Patient disposition 16 mg/kg GEN 501, Part 2 n = 42 SIRIUS n = 106 Combined N = 148 31 (74) 96 (91) 127 (86) 26 (62) 88 (83) 114 (77) Adverse event 1 (2) 5 (5) 6 (4) Physician decision 4 (10) 0 4 (3) 0 3 (3) 3 (2) Discontinued from treatment, n (%) Progressive disease Withdrawal of consent • In the combined dataset – Median (range) duration of treatment = 3. 4 (0 -20) months – Median (range) number of infusions = 12 (1 -33) • Death within 30 days of the last dose of treatment = 14 – 11 (7%) progressive disease – 3 (2%) adverse events Usmani S, ASH 2015 Abst 29

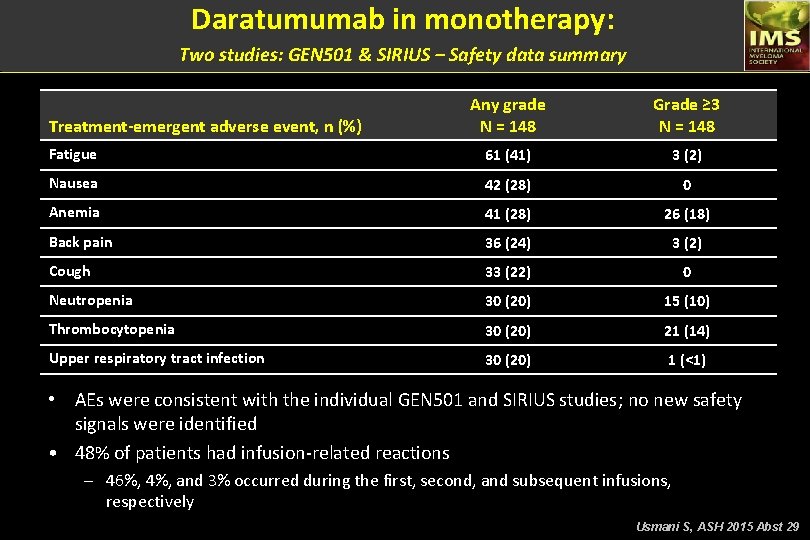
Daratumumab in monotherapy: Two studies: GEN 501 & SIRIUS – Safety data summary Any grade N = 148 Grade ≥ 3 N = 148 Fatigue 61 (41) 3 (2) Nausea 42 (28) 0 Anemia 41 (28) 26 (18) Back pain 36 (24) 3 (2) Cough 33 (22) 0 Neutropenia 30 (20) 15 (10) Thrombocytopenia 30 (20) 21 (14) Upper respiratory tract infection 30 (20) 1 (<1) Treatment-emergent adverse event, n (%) • AEs were consistent with the individual GEN 501 and SIRIUS studies; no new safety signals were identified • 48% of patients had infusion-related reactions – 46%, 4%, and 3% occurred during the first, second, and subsequent infusions, respectively Usmani S, ASH 2015 Abst 29

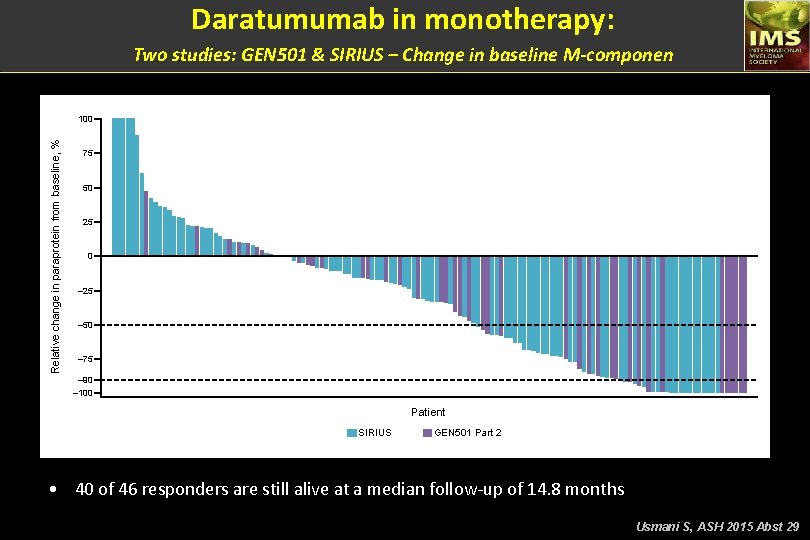
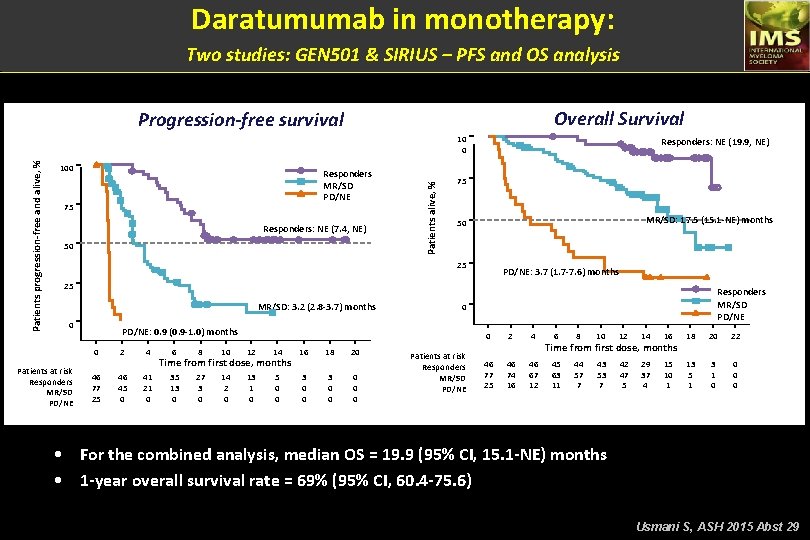
Daratumumab in monotherapy: Two studies: GEN 501 & SIRIUS – Change in baseline M-componen Relative change in paraprotein from baseline, % 100 75 50 25 0 – 25 – 50 – 75 – 90 – 100 Patient SIRIUS GEN 501 Part 2 • 40 of 46 responders are still alive at a median follow-up of 14. 8 months Usmani S, ASH 2015 Abst 29

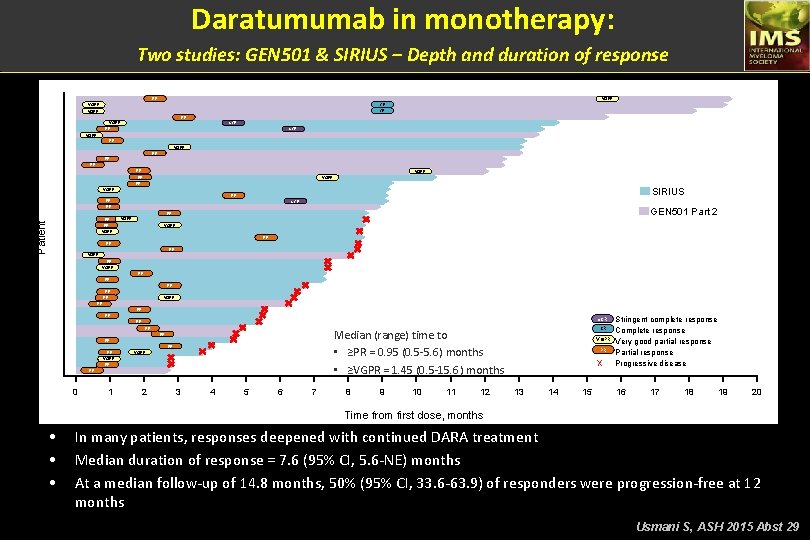
Daratumumab in monotherapy: Two studies: GEN 501 & SIRIUS – Depth and duration of response PR VGPR CR PR s. CR VGPR PR PR VGPR PR SIRIUS VGPR PR PR s. CR PR GEN 501 Part 2 PR Patient PR VGPR PR VGPR PR Median (range) time to • Median (range) time to ≥PR = 0. 95 (0. 5 -5. 6) months • • ≥VGPR = 1. 45 (0. 5 -15. 6) months PR PR VGPR PR PR 0 1 2 3 4 5 6 7 8 9 10 11 12 s. CR Stringent complete response CR Complete response VGPR Very good partial response PR Partial response X Progressive disease 13 14 15 16 17 18 19 20 Time from first dose, months • • • In many patients, responses deepened with continued DARA treatment Median duration of response = 7. 6 (95% CI, 5. 6 -NE) months At a median follow-up of 14. 8 months, 50% (95% CI, 33. 6 -63. 9) of responders were progression-free at 12 months Usmani S, ASH 2015 Abst 29

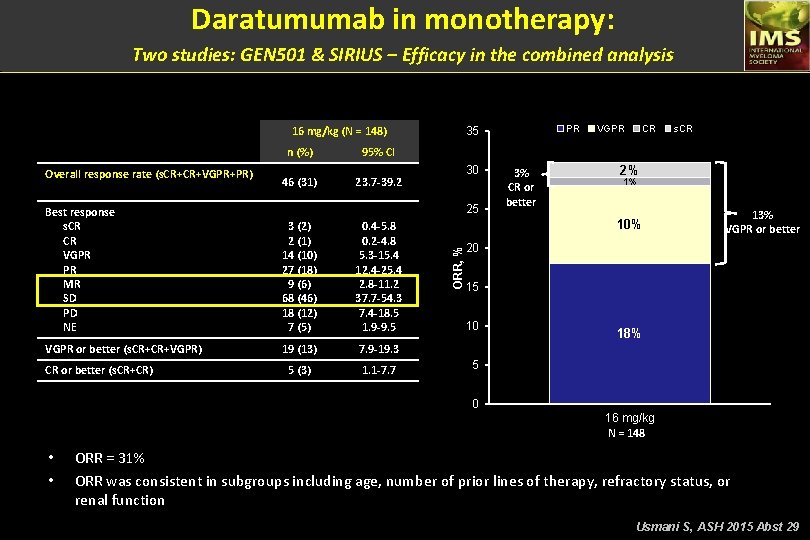
Daratumumab in monotherapy: Two studies: GEN 501 & SIRIUS – Efficacy in the combined analysis 16 mg/kg (N = 148) 95% CI 46 (31) 23. 7 -39. 2 3 (2) 2 (1) 14 (10) 27 (18) 9 (6) 68 (46) 18 (12) 7 (5) 0. 4 -5. 8 0. 2 -4. 8 5. 3 -15. 4 12. 4 -25. 4 2. 8 -11. 2 37. 7 -54. 3 7. 4 -18. 5 1. 9 -9. 5 VGPR or better (s. CR+CR+VGPR) 19 (13) 7. 9 -19. 3 5 (3) 1. 1 -7. 7 CR or better (s. CR+CR) 30 25 Best response s. CR CR VGPR PR MR SD PD NE VGPR CR s. CR ORR = 31% 3% CR or better 2% 1% 10% ORR, % Overall response rate (s. CR+CR+VGPR+PR) n (%) PR 35 13% VGPR or better 20 15 10 18% 5 0 16 mg/kg N = 148 • • ORR = 31% ORR was consistent in subgroups including age, number of prior lines of therapy, refractory status, or renal function Usmani S, ASH 2015 Abst 29

Daratumumab in monotherapy: Two studies: GEN 501 & SIRIUS – PFS and OS analysis Overall Survival Progression-free survival 100 Responders MR/SD PD/NE 75 Responders: NE (7. 4, NE) 50 Patients alive, % Patients progression-free and alive, % 10 0 Responders: NE (19. 9, NE) 75 MR/SD: 17. 5 (15. 1 -NE) months 50 25 PD/NE: 3. 7 (1. 7 -7. 6) months 25 MR/SD: 3. 2 (2. 8 -3. 7) months 0 Patients at risk Responders MR/SD PD/NE 0 PD/NE: 0. 9 (0. 9 -1. 0) months 0 46 77 25 2 46 45 0 4 41 21 0 6 8 10 12 14 Time from first dose, months 35 13 0 27 3 0 14 2 0 13 1 0 5 0 0 16 3 0 0 18 3 0 0 20 0 Responders MR/SD PD/NE Patients at risk Responders MR/SD PD/NE 0 2 4 46 77 25 46 74 16 46 67 12 6 8 10 12 14 16 18 20 22 45 63 11 44 57 7 43 53 7 42 47 5 29 37 4 15 10 1 13 5 1 3 1 0 0 Time from first dose, months • For the combined analysis, median OS = 19. 9 (95% CI, 15. 1 -NE) months • 1 -year overall survival rate = 69% (95% CI, 60. 4 -75. 6) Usmani S, ASH 2015 Abst 29

A Dose Finding Phase II Trial of Isatuximab (SAR 650984, Anti-CD 38 m. Ab) as a Single Agent in Relapsed/Refractory Multiple Myeloma Thomas Martin, 1 Joshua Richter, 2 Ravi Vij, 3 Craig Cole, 4 Djordje Atanackovic, 5 Jeffrey Zonder, 6 Jonathan Kaufman, 7 William Bensinger, 8 Meletios Dimopoulos, 9 Jesús San Miguel, 10 Todd Zimmerman, 11 Nikoletta Lendvai, 12 Parameswaran Hari, 13 Enrique Ocio, 14 Cristina Gasparetto, 15 Shaji Kumar, 16 Karl Hsu, 17 Eric Charpentier, 17 Stephen Strickland, 18 Joseph Mikhael 19 1 University of California at San Francisco, CA, USA; 2 Hackensack University Medical Center, Hackensack, NJ, USA; 3 Washington University, St. Louis, MO, USA; 4 University of Michigan Comprehensive Cancer Center, Ann Arbor, MI, USA; 5 Huntsman Cancer Institute, Salt Lake City, UT, USA; 6 Karmanos Cancer Center, Detroit, MI, USA; 7 Winship Cancer Institute of Emory University, Atlanta, GA, USA; 8 Fred Hutchinson Cancer Research Center, Seattle, WA, USA; 9 National and Kapodistrian University of Athens, Greece; 10 University of Navarra, Pamplona, Spain; 11 University of Chicago, IL, USA; 12 Memorial Sloan-Kettering Cancer Center, New York, NY, USA; 13 Medical College of Wisconsin, Milwaukee, WI, USA; 14 University Hospital of Salamanca, Spain; 15 Duke University Medical Center, Durham, NC, USA; 16 Mayo Clinic, Rochester, MN, USA; 17 Sanofi Oncology, Cambridge, MA, USA; 18 Vanderbilt-Ingram Cancer Center, Nashville, TN, USA; 19 Mayo Clinic, Scottsdale, AZ, USA Martin T, ASH 2015 Abst 509

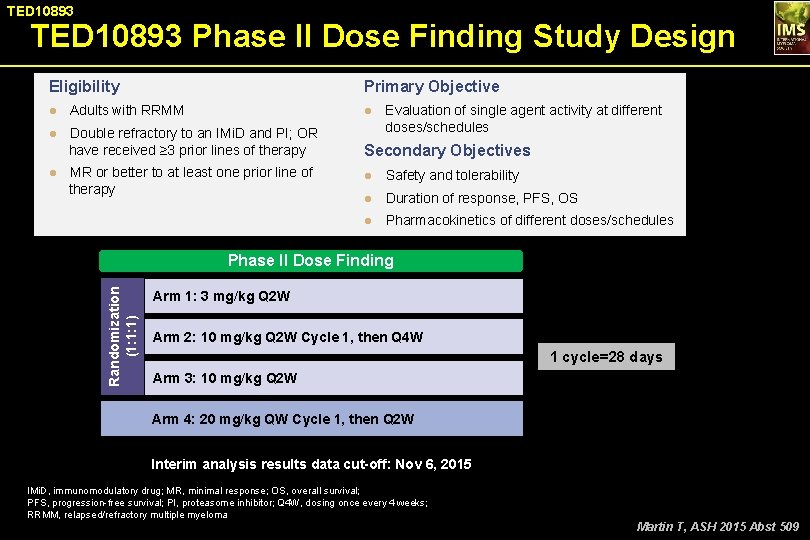
TED 10893 Phase II Dose Finding Study Design Eligibility Primary Objective ● Adults with RRMM ● Evaluation of single agent activity at different ● Double refractory to an IMi. D and PI; OR doses/schedules have received ≥ 3 prior lines of therapy Secondary Objectives ● MR or better to at least one prior line of therapy ● Safety and tolerability ● Duration of response, PFS, OS ● Pharmacokinetics of different doses/schedules Randomization (1: 1: 1) Phase II Dose Finding Arm 1: 3 mg/kg Q 2 W Arm 2: 10 mg/kg Q 2 W Cycle 1, then Q 4 W 1 cycle=28 days Arm 3: 10 mg/kg Q 2 W Arm 4: 20 mg/kg QW Cycle 1, then Q 2 W Interim analysis results data cut-off: Nov 6, 2015 IMi. D, immunomodulatory drug; MR, minimal response; OS, overall survival; PFS, progression-free survival; PI, proteasome inhibitor; Q 4 W, dosing once every 4 weeks; RRMM, relapsed/refractory multiple myeloma Martin T, ASH 2015 Abst 509

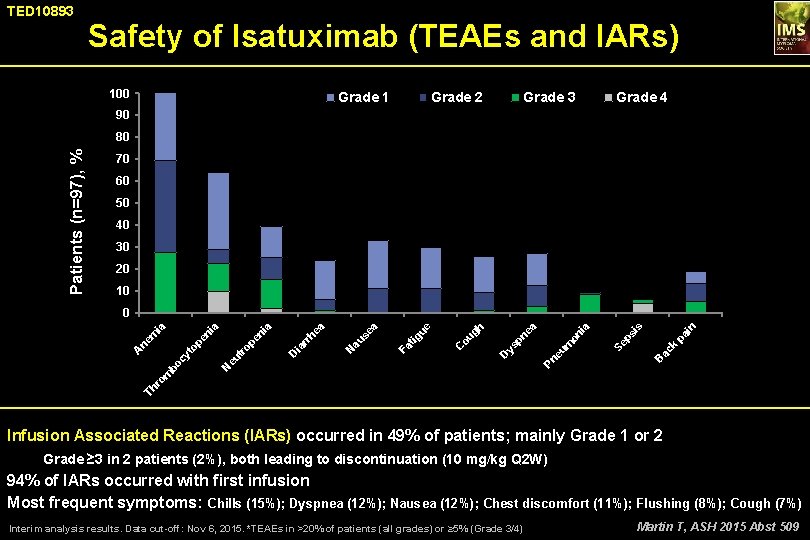
TED 10893 Safety of Isatuximab (TEAEs and IARs) 100 Grade 1 Grade 2 Grade 3 Grade 4 90 Patients (n=97), % 80 70 60 50 40 30 20 10 k pa i n is B ac ps on m Pn eu Se ia a pn e ys D C ou gh ue ig Fa t N au se a ea D ia rr h ia pe n tr o eu N Th r om bo cy t A ne op e m ni a ia 0 Infusion Associated Reactions (IARs) occurred in 49% of patients; mainly Grade 1 or 2 Grade ≥ 3 in 2 patients (2%), both leading to discontinuation (10 mg/kg Q 2 W) 94% of IARs occurred with first infusion Most frequent symptoms: Chills (15%); Dyspnea (12%); Nausea (12%); Chest discomfort (11%); Flushing (8%); Cough (7%) Interim analysis results. Data cut-off: Nov 6, 2015. *TEAEs in >20% of patients (all grades) or ≥ 5% (Grade 3/4) Martin T, ASH 2015 Abst 509

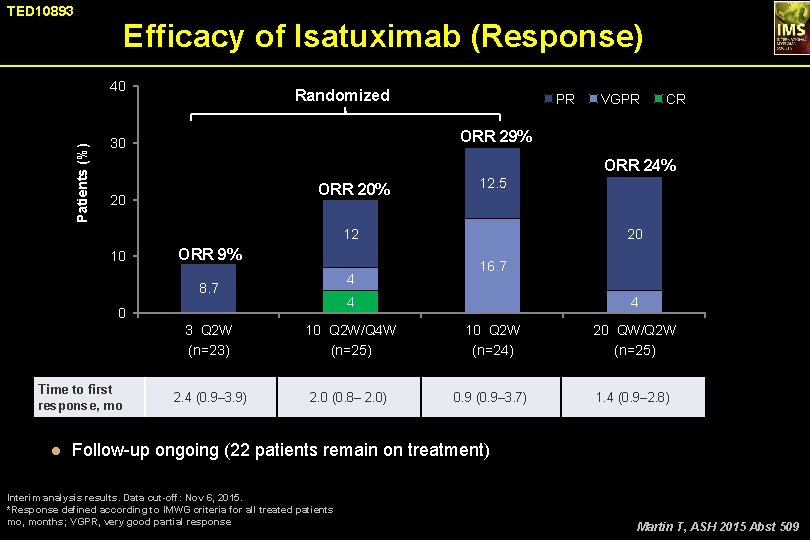
TED 10893 Efficacy of Isatuximab (Response) Patients (%) 40 Randomized PR VGPR CR ORR 29% 30 ORR 24% ORR 20% 20 12. 5 12 10 ORR 9% 4 8. 7 16. 7 4 0 Time to first response, mo 20 3 Q 2 W (n=23) 10 Q 2 W/Q 4 W (n=25) 2. 4 (0. 9– 3. 9) 2. 0 (0. 8– 2. 0) 4 10 Q 2 W (n=24) 0. 9 (0. 9– 3. 7) 20 QW/Q 2 W (n=25) 1. 4 (0. 9– 2. 8) ● Follow-up ongoing (22 patients remain on treatment) Interim analysis results. Data cut-off: Nov 6, 2015. *Response defined according to IMWG criteria for all treated patients mo, months; VGPR, very good partial response Martin T, ASH 2015 Abst 509

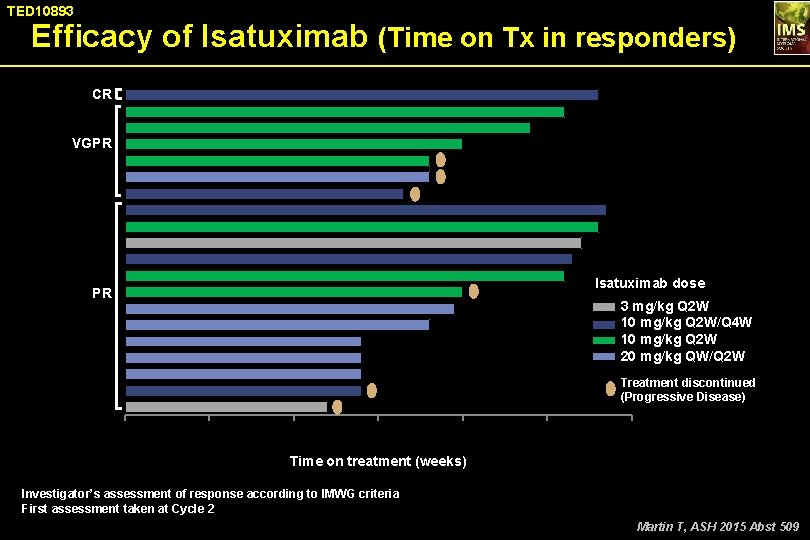
TED 10893 Efficacy of Isatuximab (Time on Tx in responders) CR VGPR Isatuximab dose PR 3 mg/kg Q 2 W 10 mg/kg Q 2 W/Q 4 W 10 mg/kg Q 2 W 20 mg/kg QW/Q 2 W Treatment discontinued (Progressive Disease) 0 10 20 30 40 50 60 Time on treatment (weeks) Investigator’s assessment of response according to IMWG criteria First assessment taken at Cycle 2 Martin T, ASH 2015 Abst 509

TED 10893 Summary • Isatuximab demonstrated single-agent activity in heavily pretreated RRMM patients – Median 5 (2– 14) prior lines of therapy – 88% double refractory; 41% quadruple refractory • The preliminary ORR was 24– 29% in the two highest dose intensity cohorts, consistent with Phase I results • Isatuximab was well tolerated – – Safety profile was similar across different treatment arms No new safety signals emerged compared with Phase I Infusion reactions predominantly Grade 1/2 in intensity; majority in Cycle 1 Infusion durations are shorter following the first infusion • Optimal dose and schedule to be evaluated in an expanded population • Isatuximab will be advanced to Phase III Martin T, ASH 2015 Abst 509

Impact of Prior Treatment on Patients With Relapsed Multiple Myeloma Treated With Carfilzomib and Dexamethasone vs Bortezomib and Dexamethasone in a Subgroup Analysis of the Phase 3 ENDEAVOR Study (NCT 01568866) Philippe Moreau, 1 Douglas Joshua, 2 Wee-Joo Chng, 3 Antonio Palumbo, 4 Hartmut Goldschmidt, 5 Roman Hájek, 6 Thierry Facon, 7 Heinz Ludwig, 8 Ludek Pour, 9 Ruben Niesvizky, 10 Albert Oriol, 11 Laura Rosiñol, 12 Aleksandr Suvorov, 13 Gianluca Gaidano, 14 Tomas Pika, 15 Katja Weisel, 16 Vesselina Goranova-Marinova, 17 Heidi H. Gillenwater, 18 Nehal Mohamed, 18 Shibao Feng, 18 Meletios A. Dimopoulos 19 Moreau P, ASH 2015 Abst 729

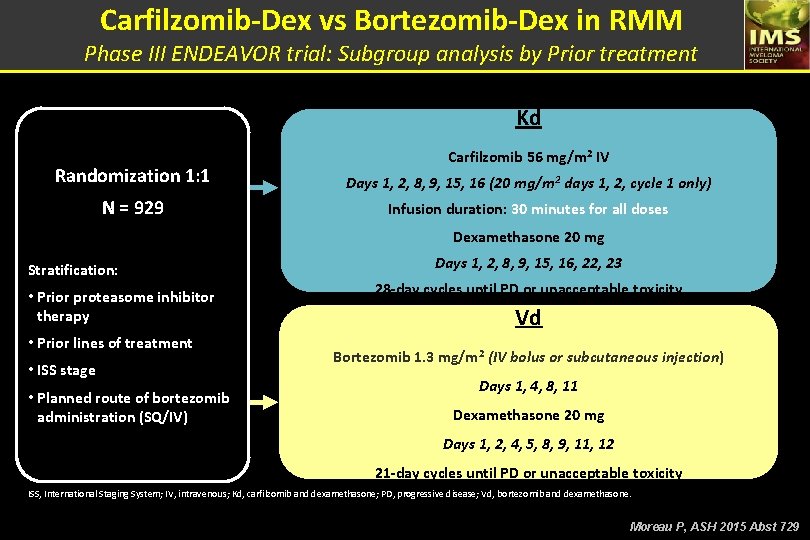
Carfilzomib-Dex vs Bortezomib-Dex in RMM Phase III ENDEAVOR trial: Subgroup analysis by Prior treatment Kd Carfilzomib 56 mg/m 2 IV Randomization 1: 1 Days 1, 2, 8, 9, 15, 16 (20 mg/m 2 days 1, 2, cycle 1 only) N = 929 Infusion duration: 30 minutes for all doses Dexamethasone 20 mg Stratification: • Prior proteasome inhibitor therapy • Prior lines of treatment • ISS stage • Planned route of bortezomib administration (SQ/IV) Days 1, 2, 8, 9, 15, 16, 22, 23 28 -day cycles until PD or unacceptable toxicity Vd Bortezomib 1. 3 mg/m 2 (IV bolus or subcutaneous injection) Days 1, 4, 8, 11 Dexamethasone 20 mg Days 1, 2, 4, 5, 8, 9, 11, 12 21 -day cycles until PD or unacceptable toxicity ISS, International Staging System; IV, intravenous; Kd, carfilzomib and dexamethasone; PD, progressive disease; Vd, bortezomib and dexamethasone. Moreau P, ASH 2015 Abst 729

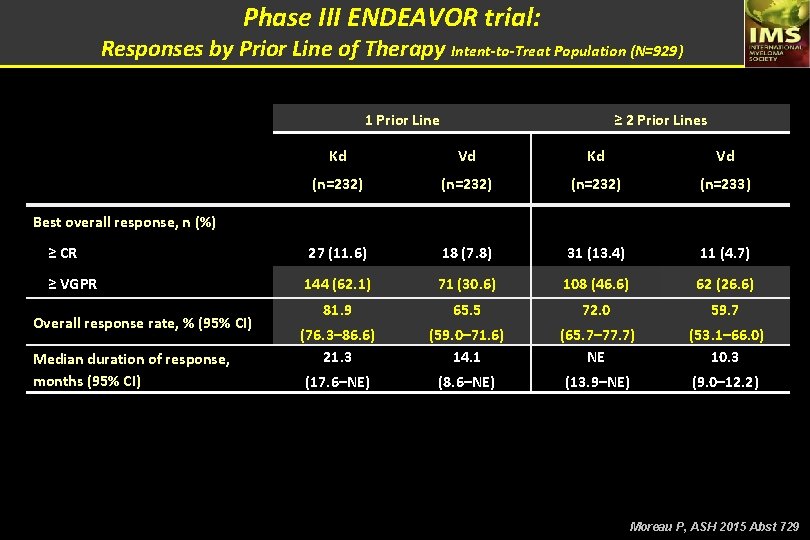
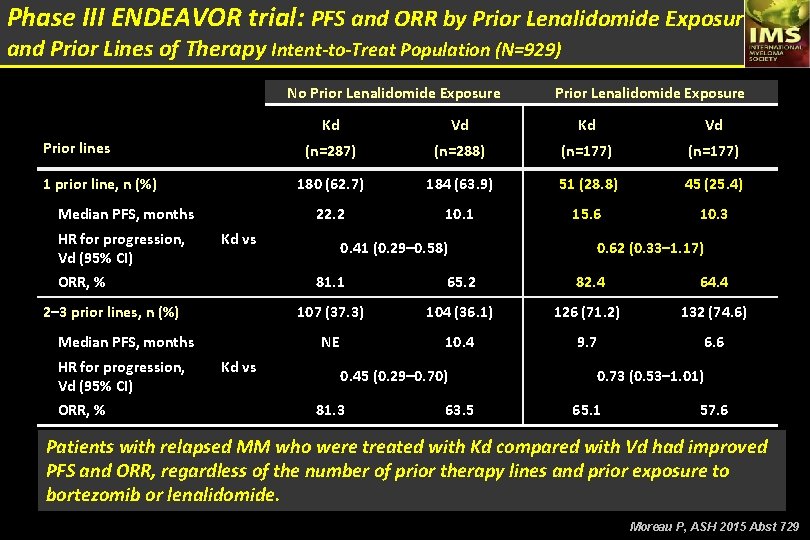
Phase III ENDEAVOR trial: Responses by Prior Line of Therapy Intent-to-Treat Population (N=929) 1 Prior Line ≥ 2 Prior Lines Kd Vd (n=232) (n=233) ≥ CR 27 (11. 6) 18 (7. 8) 31 (13. 4) 11 (4. 7) ≥ VGPR 144 (62. 1) 71 (30. 6) 108 (46. 6) 62 (26. 6) 81. 9 65. 5 72. 0 59. 7 (76. 3– 86. 6) 21. 3 (59. 0– 71. 6) 14. 1 (65. 7– 77. 7) NE (53. 1– 66. 0) 10. 3 (17. 6–NE) (8. 6–NE) (13. 9–NE) (9. 0– 12. 2) Best overall response, n (%) Overall response rate, % (95% CI) Median duration of response, months (95% CI) Moreau P, ASH 2015 Abst 729

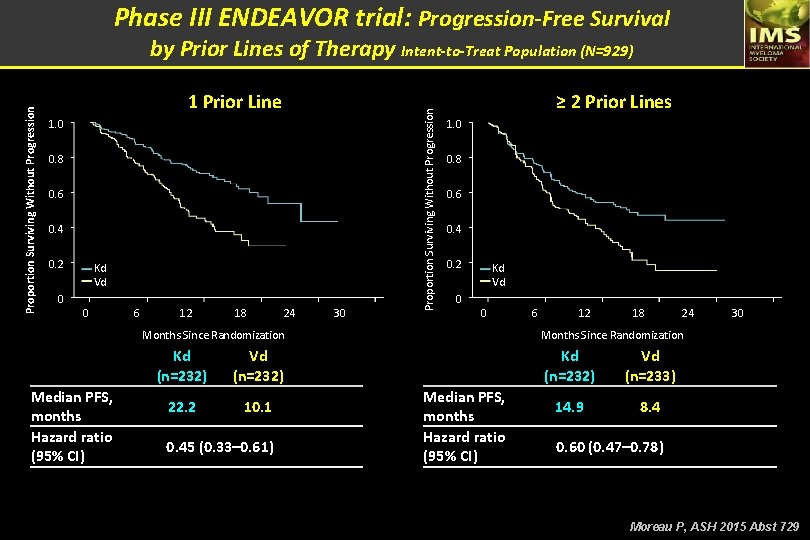
Phase III ENDEAVOR trial: Progression-Free Survival 1 Prior Line 1. 0 0. 8 0. 6 0. 4 0. 2 0 Kd Vd 0 6 12 18 24 30 Proportion Surviving Without Progression by Prior Lines of Therapy Intent-to-Treat Population (N=929) ≥ 2 Prior Lines 1. 0 0. 8 0. 6 0. 4 0. 2 Kd Vd 0 0 Months Since Randomization Median PFS, months Hazard ratio (95% CI) Kd (n=232) Vd (n=232) 22. 2 10. 1 0. 45 (0. 33– 0. 61) 6 12 18 24 30 Months Since Randomization Median PFS, months Hazard ratio (95% CI) Kd (n=232) Vd (n=233) 14. 9 8. 4 0. 60 (0. 47– 0. 78) Moreau P, ASH 2015 Abst 729

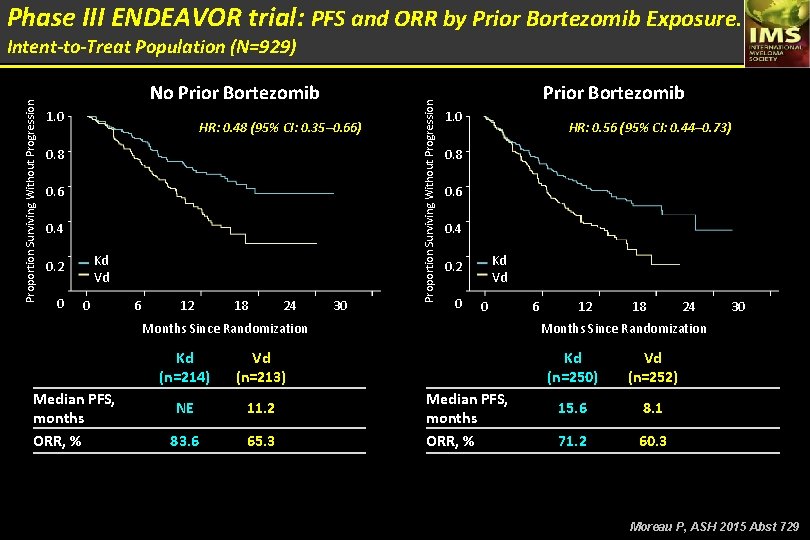
Phase III ENDEAVOR trial: PFS and ORR by Prior Bortezomib Exposure. No Prior Bortezomib 1. 0 HR: 0. 48 (95% CI: 0. 35– 0. 66) 0. 8 0. 6 0. 4 Kd Vd 0. 2 0 0 6 12 18 24 30 Proportion Surviving Without Progression Intent-to-Treat Population (N=929) Prior Bortezomib 1. 0 HR: 0. 56 (95% CI: 0. 44– 0. 73) 0. 8 0. 6 0. 4 Kd Vd 0. 2 0 0 Months Since Randomization Median PFS, months ORR, % Kd (n=214) Vd (n=213) NE 11. 2 83. 6 65. 3 6 12 18 24 30 Months Since Randomization Median PFS, months ORR, % Kd (n=250) Vd (n=252) 15. 6 8. 1 71. 2 60. 3 Moreau P, ASH 2015 Abst 729

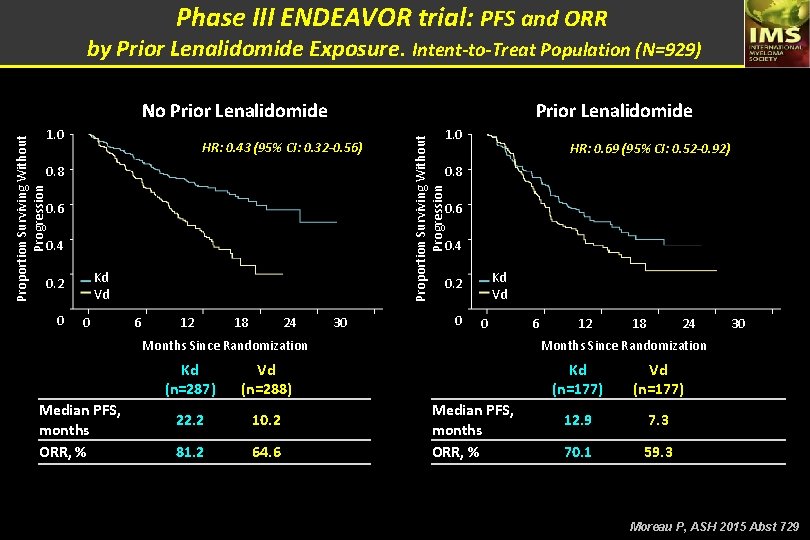
Phase III ENDEAVOR trial: PFS and ORR by Prior Lenalidomide Exposure. Intent-to-Treat Population (N=929) No Prior Lenalidomide HR: 0. 43 (95% CI: 0. 32 -0. 56) 0. 8 HR: 0. 69 (95% CI: 0. 52 -0. 92) 0. 8 0. 6 0. 4 Kd Vd 0. 2 0 1. 0 Proportion Surviving Without Progression 1. 0 Prior Lenalidomide 0 Kd Vd 0. 2 6 12 18 24 30 0 0 Months Since Randomization Median PFS, months ORR, % Kd (n=287) Vd (n=288) 22. 2 10. 2 81. 2 64. 6 6 12 18 24 30 Months Since Randomization Median PFS, months ORR, % Kd (n=177) Vd (n=177) 12. 9 7. 3 70. 1 59. 3 Moreau P, ASH 2015 Abst 729

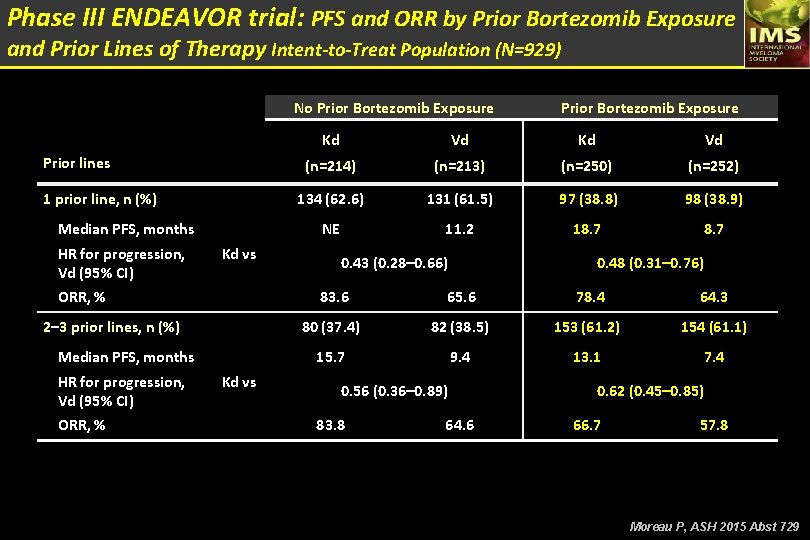
Phase III ENDEAVOR trial: PFS and ORR by Prior Bortezomib Exposure and Prior Lines of Therapy Intent-to-Treat Population (N=929) No Prior Bortezomib Exposure Prior lines 1 prior line, n (%) Median PFS, months HR for progression, Kd vs Vd (95% CI) ORR, % 2– 3 prior lines, n (%) Median PFS, months HR for progression, Kd vs Vd (95% CI) ORR, % Prior Bortezomib Exposure Kd Vd (n=214) (n=213) (n=250) (n=252) 134 (62. 6) 131 (61. 5) 97 (38. 8) 98 (38. 9) NE 11. 2 18. 7 0. 43 (0. 28– 0. 66) 0. 48 (0. 31– 0. 76) 83. 6 65. 6 78. 4 64. 3 80 (37. 4) 82 (38. 5) 153 (61. 2) 154 (61. 1) 15. 7 9. 4 13. 1 7. 4 0. 56 (0. 36– 0. 89) 83. 8 64. 6 0. 62 (0. 45– 0. 85) 66. 7 57. 8 Moreau P, ASH 2015 Abst 729

Phase III ENDEAVOR trial: PFS and ORR by Prior Lenalidomide Exposure and Prior Lines of Therapy Intent-to-Treat Population (N=929) No Prior Lenalidomide Exposure Prior lines 1 prior line, n (%) Median PFS, months Kd Vd (n=287) (n=288) (n=177) 180 (62. 7) 184 (63. 9) 51 (28. 8) 45 (25. 4) 22. 2 10. 1 15. 6 10. 3 HR for progression, Kd vs Vd (95% CI) ORR, % 2– 3 prior lines, n (%) Median PFS, months HR for progression, Kd vs Vd (95% CI) ORR, % Prior Lenalidomide Exposure 0. 41 (0. 29– 0. 58) 0. 62 (0. 33– 1. 17) 81. 1 65. 2 82. 4 64. 4 107 (37. 3) 104 (36. 1) 126 (71. 2) 132 (74. 6) NE 10. 4 9. 7 6. 6 0. 45 (0. 29– 0. 70) 81. 3 63. 5 0. 73 (0. 53– 1. 01) 65. 1 57. 6 Patients with relapsed MM who were treated with Kd compared with Vd had improved PFS and ORR, regardless of the number of prior therapy lines and prior exposure to bortezomib or lenalidomide. Moreau P, ASH 2015 Abst 729

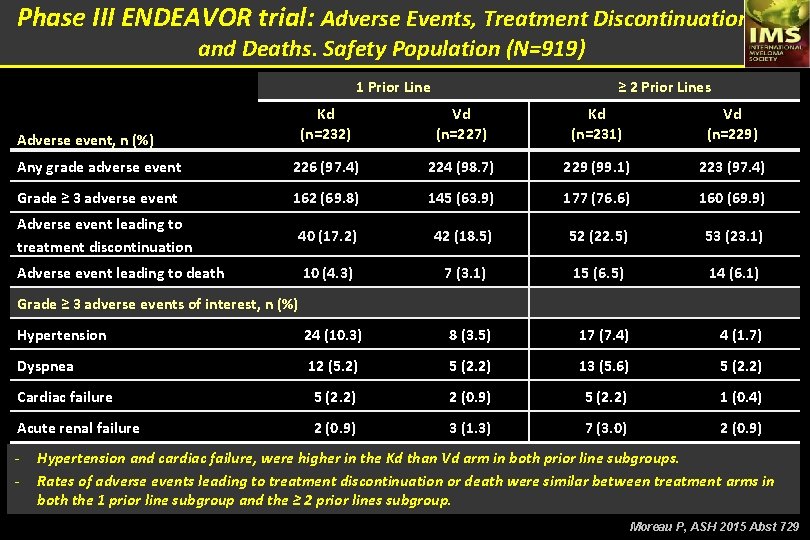
Phase III ENDEAVOR trial: Adverse Events, Treatment Discontinuations, and Deaths. Safety Population (N=919) 1 Prior Line ≥ 2 Prior Lines Kd (n=232) Vd (n=227) Kd (n=231) Vd (n=229) Any grade adverse event 226 (97. 4) 224 (98. 7) 229 (99. 1) 223 (97. 4) Grade ≥ 3 adverse event 162 (69. 8) 145 (63. 9) 177 (76. 6) 160 (69. 9) Adverse event leading to treatment discontinuation 40 (17. 2) 42 (18. 5) 52 (22. 5) 53 (23. 1) Adverse event leading to death 10 (4. 3) 7 (3. 1) 15 (6. 5) 14 (6. 1) Hypertension 24 (10. 3) 8 (3. 5) 17 (7. 4) 4 (1. 7) Dyspnea 12 (5. 2) 5 (2. 2) 13 (5. 6) 5 (2. 2) Cardiac failure 5 (2. 2) 2 (0. 9) 5 (2. 2) 1 (0. 4) Acute renal failure 2 (0. 9) 3 (1. 3) 7 (3. 0) 2 (0. 9) Adverse event, n (%) Grade ≥ 3 adverse events of interest, n (%) - Hypertension and cardiac failure, were higher in the Kd than Vd arm in both prior line subgroups. Rates of adverse events leading to treatment discontinuation or death were similar between treatment arms in both the 1 prior line subgroup and the ≥ 2 prior lines subgroup. Moreau P, ASH 2015 Abst 729

Efficacy and safety of Carfilzomib and Dexamethasone vs Bortezomib and Dexamethasone in Patients with Relapsed Multiple Myeloma based on Cytogenetic Risk Status: Subgroup Analysis from the Phase 3 ENDEAVOR Study (NCT 01568866) Wee Joo Chng, Hartmut Goldschmidt, Meletios A Dimopoulos, Philippe Moreau, Douglas Joshua, Antonio Palumbo, Thierry Facon, Heinz Ludwig, Ludek Pour, Ruben Niesvizky, Albert Oriol, Laura Rosiñol, Aleksandr Suvorov, Gianluca Gaidano, Tomas Pika, Katja Weisel, Vesselina Goranova-Marinova, Heidi H. Gillenwater, Nehal Mohamed, Shibao Feng and Roman Hájek. Chng WJ, ASH 2015 Abst 30

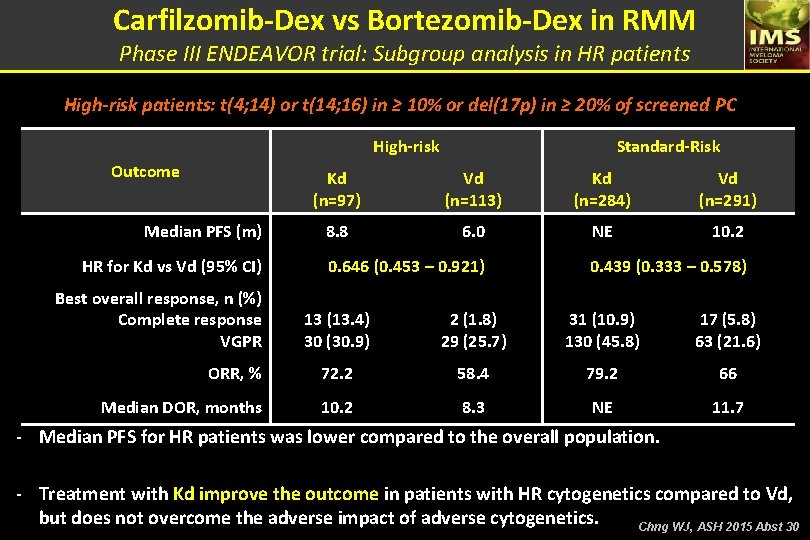
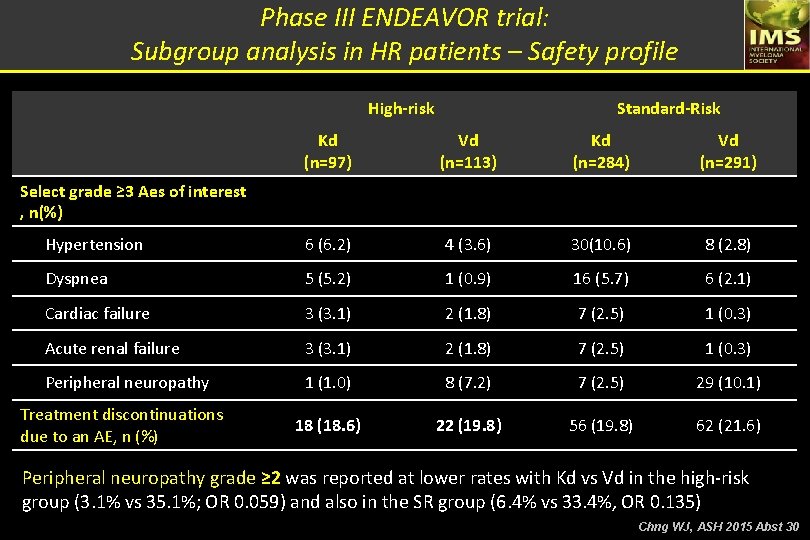
Carfilzomib-Dex vs Bortezomib-Dex in RMM Phase III ENDEAVOR trial: Subgroup analysis in HR patients High-risk patients: t(4; 14) or t(14; 16) in ≥ 10% or del(17 p) in ≥ 20% of screened PC High-risk Outcome Median PFS (m) HR for Kd vs Vd (95% CI) Best overall response, n (%) Complete response VGPR Standard-Risk Kd (n=97) Vd (n=113) Kd (n=284) Vd (n=291) 8. 8 6. 0 NE 10. 2 0. 646 (0. 453 – 0. 921) 0. 439 (0. 333 – 0. 578) 13 (13. 4) 30 (30. 9) 2 (1. 8) 29 (25. 7) 31 (10. 9) 130 (45. 8) 17 (5. 8) 63 (21. 6) ORR, % 72. 2 58. 4 79. 2 66 Median DOR, months 10. 2 8. 3 NE 11. 7 - Median PFS for HR patients was lower compared to the overall population. - Treatment with Kd improve the outcome in patients with HR cytogenetics compared to Vd, but does not overcome the adverse impact of adverse cytogenetics. Chng WJ, ASH 2015 Abst 30

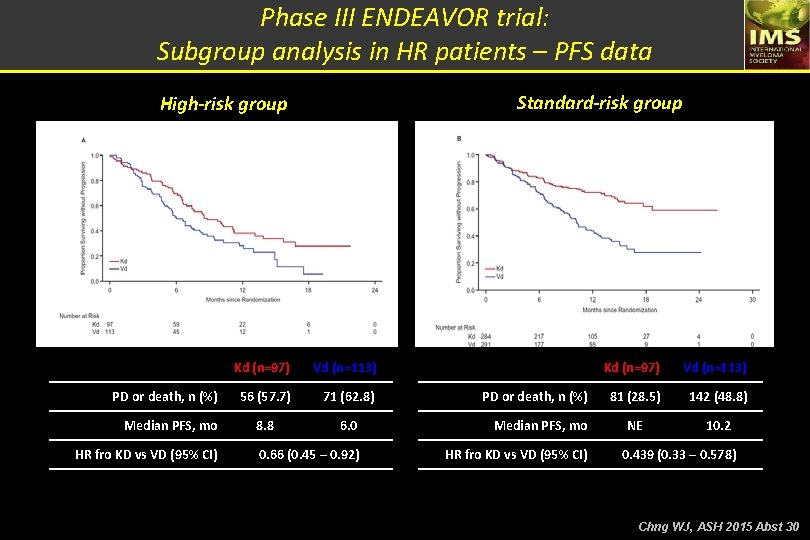
Phase III ENDEAVOR trial: Subgroup analysis in HR patients – PFS data Standard-risk group High-risk group PD or death, n (%) Median PFS, mo HR fro KD vs VD (95% CI) Kd (n=97) Vd (n=113) 56 (57. 7) 71 (62. 8) 8. 8 6. 0 Median PFS, mo 0. 66 (0. 45 – 0. 92) HR fro KD vs VD (95% CI) PD or death, n (%) Kd (n=97) Vd (n=113) 81 (28. 5) 142 (48. 8) NE 10. 2 0. 439 (0. 33 – 0. 578) Chng WJ, ASH 2015 Abst 30

Phase III ENDEAVOR trial: Subgroup analysis in HR patients – Safety profile High-risk Standard-Risk Kd (n=97) Vd (n=113) Kd (n=284) Vd (n=291) Hypertension 6 (6. 2) 4 (3. 6) 30(10. 6) 8 (2. 8) Dyspnea 5 (5. 2) 1 (0. 9) 16 (5. 7) 6 (2. 1) Cardiac failure 3 (3. 1) 2 (1. 8) 7 (2. 5) 1 (0. 3) Acute renal failure 3 (3. 1) 2 (1. 8) 7 (2. 5) 1 (0. 3) Peripheral neuropathy 1 (1. 0) 8 (7. 2) 7 (2. 5) 29 (10. 1) 18 (18. 6) 22 (19. 8) 56 (19. 8) 62 (21. 6) Select grade ≥ 3 Aes of interest , n(%) Treatment discontinuations due to an AE, n (%) Peripheral neuropathy grade ≥ 2 was reported at lower rates with Kd vs Vd in the high-risk group (3. 1% vs 35. 1%; OR 0. 059) and also in the SR group (6. 4% vs 33. 4%, OR 0. 135) Chng WJ, ASH 2015 Abst 30

Phase III ENDEAVOR trial: Subgroup analysis in HR patients – Conclusions § Median PFS for patients with high risk cytogenetics was lower compared with the overall population. § Patients treated with Kd has a clinically meaningful improvement in PFS compared with Vd in patients with high- or standard cytogenetics. § Higher response rates, a greater depth of response, a longer DOR were also observed with Kd vs Vd across cytogenetic subgroups. § Kd had a favorable benefit-risk profile in patients with high-risk relapsed MM and was superior to Vd, regardless of baseline cytogenetic risk status. Chng WJ, ASH 2015 Abst 30

Efficacy and Safety of Carfilzomib, Lenalidomide, and Dexamethasone vs Lenalidomide and Dexamethasone in Patients With Relapsed Multiple Myeloma Based on Cytogenetic Risk Status: Subgroup Analysis From the Phase 3 Study ASPIRE (NCT 01080391) Hervé Avet-Loiseau, Rafael Fonseca, David Siegel, Meletios A. Dimopoulos, Ivan Špička, Tamás Masszi, Roman Hájek, Laura Rosiñol, Vesselina Goranova-Marinova, Georgi Mihaylov, Vladimír Maisnar, Maria-Victoria Mateos, Michael Wang, Ruben Niesvizky, Albert Oriol, Andrzej Jakubowiak, Jiri Minarik, Antonio Palumbo, William Bensinger, Vishal Kukreti, Dina Ben-Yehuda, Margaret Tonda, Mihaela Obreja, Philippe Moreau. Avet Loiseau H, ASH 2015 Abst 731

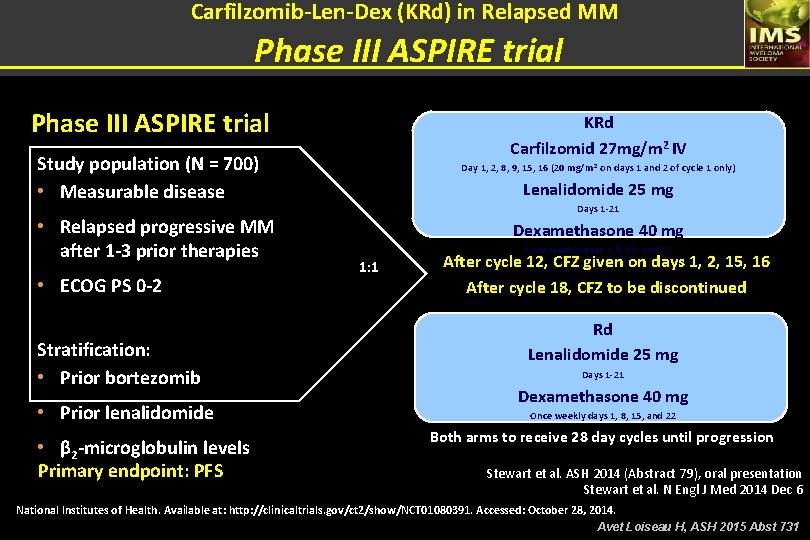
Carfilzomib-Len-Dex (KRd) in Relapsed MM Phase III ASPIRE trial KRd Carfilzomid 27 mg/m 2 IV Study population (N = 700) • Measurable disease • Relapsed progressive MM after 1 -3 prior therapies • ECOG PS 0 -2 Stratification: • Prior bortezomib • Prior lenalidomide • β 2 -microglobulin levels Primary endpoint: PFS Day 1, 2, 8, 9, 15, 16 (20 mg/m 2 on days 1 and 2 of cycle 1 only) Lenalidomide 25 mg Days 1 -21 Dexamethasone 40 mg Once weekly days 1, 8, 15, and 22 1: 1 After cycle 12, CFZ given on days 1, 2, 15, 16 After cycle 18, CFZ to be discontinued Rd Lenalidomide 25 mg Days 1 -21 Dexamethasone 40 mg Once weekly days 1, 8, 15, and 22 Both arms to receive 28 day cycles until progression Stewart et al. ASH 2014 (Abstract 79), oral presentation Stewart et al. N Engl J Med 2014 Dec 6 National Institutes of Health. Available at: http: //clinicaltrials. gov/ct 2/show/NCT 01080391. Accessed: October 28, 2014. Avet Loiseau H, ASH 2015 Abst 731

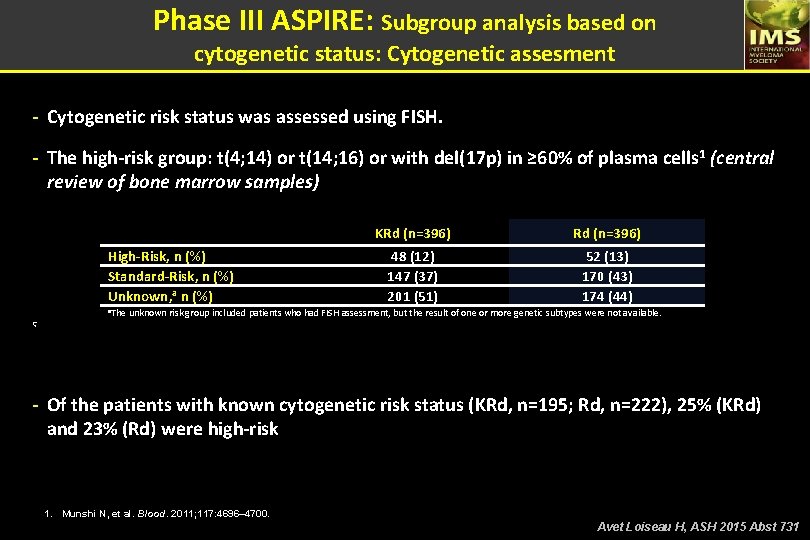
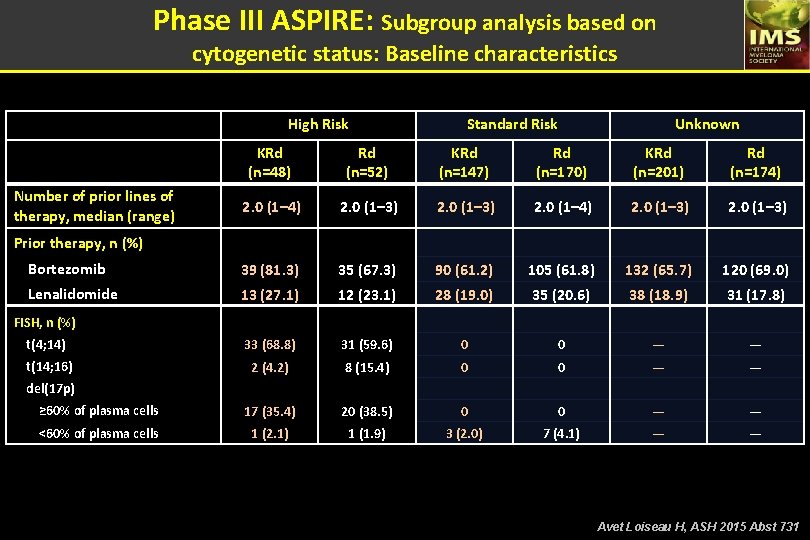
Phase III ASPIRE: Subgroup analysis based on cytogenetic status: Cytogenetic assesment - Cytogenetic risk status was assessed using FISH. - The high-risk group: t(4; 14) or t(14; 16) or with del(17 p) in ≥ 60% of plasma cells 1 (central review of bone marrow samples) High-Risk, n (%) Standard-Risk, n (%) Unknown, a n (%) KRd (n=396) 48 (12) 147 (37) 201 (51) 52 (13) 170 (43) 174 (44) a. The unknown risk group included patients who had FISH assessment, but the result of one or more genetic subtypes were not available. Ç - Of the patients with known cytogenetic risk status (KRd, n=195; Rd, n=222), 25% (KRd) and 23% (Rd) were high-risk 1. Munshi N, et al. Blood. 2011; 117: 4696– 4700. Avet Loiseau H, ASH 2015 Abst 731

Phase III ASPIRE: Subgroup analysis based on cytogenetic status: Baseline characteristics High Risk Standard Risk Unknown KRd (n=48) Rd (n=52) KRd (n=147) Rd (n=170) KRd (n=201) Rd (n=174) 2. 0 (1– 3) 2. 0 (1– 4) 2. 0 (1– 3) Bortezomib 39 (81. 3) 35 (67. 3) 90 (61. 2) 105 (61. 8) 132 (65. 7) 120 (69. 0) Lenalidomide 13 (27. 1) 12 (23. 1) 28 (19. 0) 35 (20. 6) 38 (18. 9) 31 (17. 8) t(4; 14) 33 (68. 8) 31 (59. 6) 0 0 — — t(14; 16) 2 (4. 2) 8 (15. 4) 0 0 — — ≥ 60% of plasma cells 17 (35. 4) 20 (38. 5) 0 0 — — <60% of plasma cells 1 (2. 1) 1 (1. 9) 3 (2. 0) 7 (4. 1) — — Number of prior lines of therapy, median (range) Prior therapy, n (%) FISH, n (%) del(17 p) Avet Loiseau H, ASH 2015 Abst 731

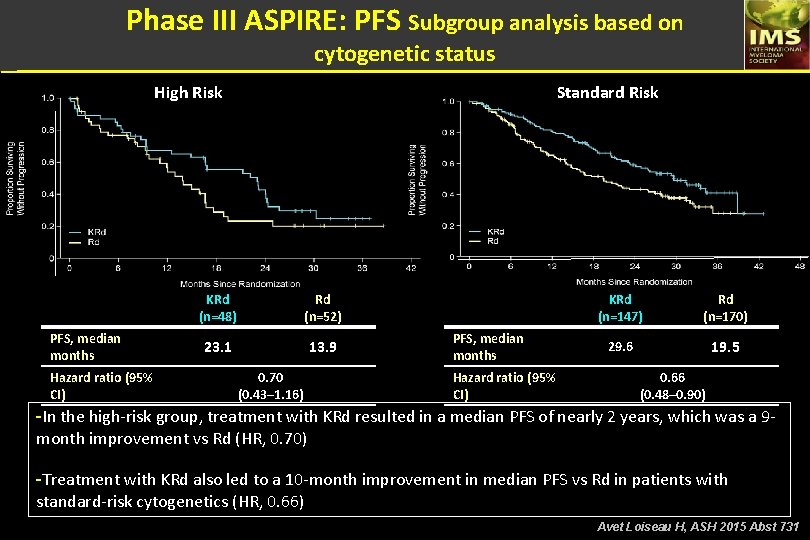
Phase III ASPIRE: PFS Subgroup analysis based on cytogenetic status High Risk PFS, median months Hazard ratio (95% CI) Standard Risk KRd (n=48) Rd (n=52) 23. 1 13. 9 0. 70 (0. 43– 1. 16) PFS, median months Hazard ratio (95% CI) KRd (n=147) Rd (n=170) 29. 6 19. 5 0. 66 (0. 48– 0. 90) -In the high-risk group, treatment with KRd resulted in a median PFS of nearly 2 years, which was a 9 month improvement vs Rd (HR, 0. 70) -Treatment with KRd also led to a 10 -month improvement in median PFS vs Rd in patients with standard-risk cytogenetics (HR, 0. 66) Avet Loiseau H, ASH 2015 Abst 731

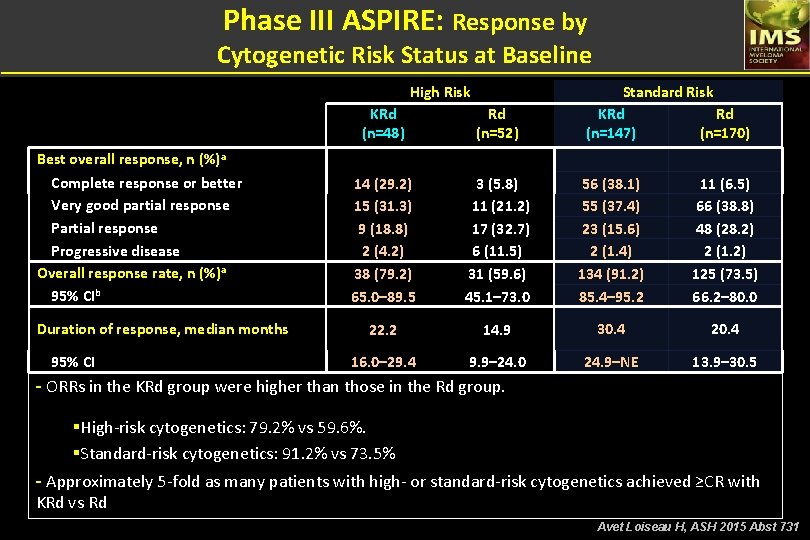
Phase III ASPIRE: Response by Cytogenetic Risk Status at Baseline High Risk Best overall response, n (%)a Complete response or better Very good partial response Progressive disease Overall response rate, n (%)a 95% CIb Duration of response, median months 95% CI Standard Risk KRd Rd (n=147) (n=170) KRd (n=48) Rd (n=52) 14 (29. 2) 15 (31. 3) 9 (18. 8) 2 (4. 2) 38 (79. 2) 65. 0– 89. 5 3 (5. 8) 11 (21. 2) 17 (32. 7) 6 (11. 5) 31 (59. 6) 45. 1– 73. 0 56 (38. 1) 55 (37. 4) 23 (15. 6) 2 (1. 4) 134 (91. 2) 85. 4– 95. 2 11 (6. 5) 66 (38. 8) 48 (28. 2) 2 (1. 2) 125 (73. 5) 66. 2– 80. 0 22. 2 14. 9 30. 4 20. 4 16. 0– 29. 4 9. 9– 24. 0 24. 9–NE 13. 9– 30. 5 - ORRs in the KRd group were higher than those in the Rd group. §High-risk cytogenetics: 79. 2% vs 59. 6%. §Standard-risk cytogenetics: 91. 2% vs 73. 5% - Approximately 5 -fold as many patients with high- or standard-risk cytogenetics achieved ≥CR with KRd vs Rd Avet Loiseau H, ASH 2015 Abst 731

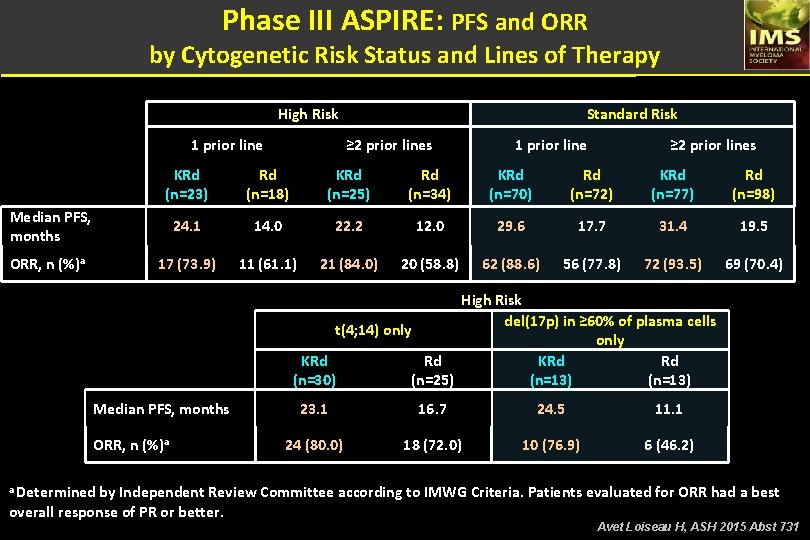
Phase III ASPIRE: PFS and ORR by Cytogenetic Risk Status and Lines of Therapy High Risk 1 prior line ≥ 2 prior lines KRd (n=23) Rd (n=18) KRd (n=25) Rd (n=34) KRd (n=70) Rd (n=72) KRd (n=77) Rd (n=98) 24. 1 14. 0 22. 2 12. 0 29. 6 17. 7 31. 4 19. 5 17 (73. 9) 11 (61. 1) 21 (84. 0) 20 (58. 8) 62 (88. 6) 56 (77. 8) 72 (93. 5) 69 (70. 4) Median PFS, months ORR, n (%)a Standard Risk High Risk del(17 p) in ≥ 60% of plasma cells t(4; 14) only KRd Rd (n=30) (n=25) (n=13) Median PFS, months ORR, n (%)a 23. 1 16. 7 24. 5 11. 1 24 (80. 0) 18 (72. 0) 10 (76. 9) 6 (46. 2) a Determined by Independent Review Committee according to IMWG Criteria. Patients evaluated for ORR had a best overall response of PR or better. Avet Loiseau H, ASH 2015 Abst 731

Phase 1 study of the Novel Spindle Protein Inhibitor Filanesib + Carfilzomib in Patients with relapsed and/or refractory Multiple Myeloma (RRMM) Jatin Shah, Lei Feng, Sheeba K. Thomas, Donna Weber, Michael Wang, Elisabet E. Manasanch, Kathleen Mendoza, Robert Z. Orlowski. Shah J, ASH 2015 Abst 376

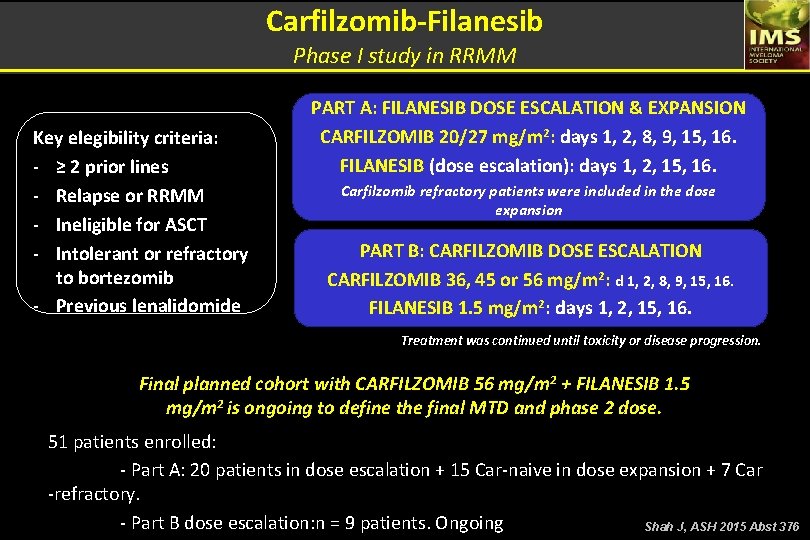
Carfilzomib-Filanesib Phase I study in RRMM Key elegibility criteria: - ≥ 2 prior lines - Relapse or RRMM - Ineligible for ASCT - Intolerant or refractory to bortezomib - Previous lenalidomide PART A: FILANESIB DOSE ESCALATION & EXPANSION CARFILZOMIB 20/27 mg/m 2: days 1, 2, 8, 9, 15, 16. FILANESIB (dose escalation): days 1, 2, 15, 16. Carfilzomib refractory patients were included in the dose expansion PART B: CARFILZOMIB DOSE ESCALATION CARFILZOMIB 36, 45 or 56 mg/m 2: d 1, 2, 8, 9, 15, 16. FILANESIB 1. 5 mg/m 2: days 1, 2, 15, 16. Treatment was continued until toxicity or disease progression. Final planned cohort with CARFILZOMIB 56 mg/m 2 + FILANESIB 1. 5 mg/m 2 is ongoing to define the final MTD and phase 2 dose. 51 patients enrolled: - Part A: 20 patients in dose escalation + 15 Car-naive in dose expansion + 7 Car -refractory. - Part B dose escalation: n = 9 patients. Ongoing Shah J, ASH 2015 Abst 376

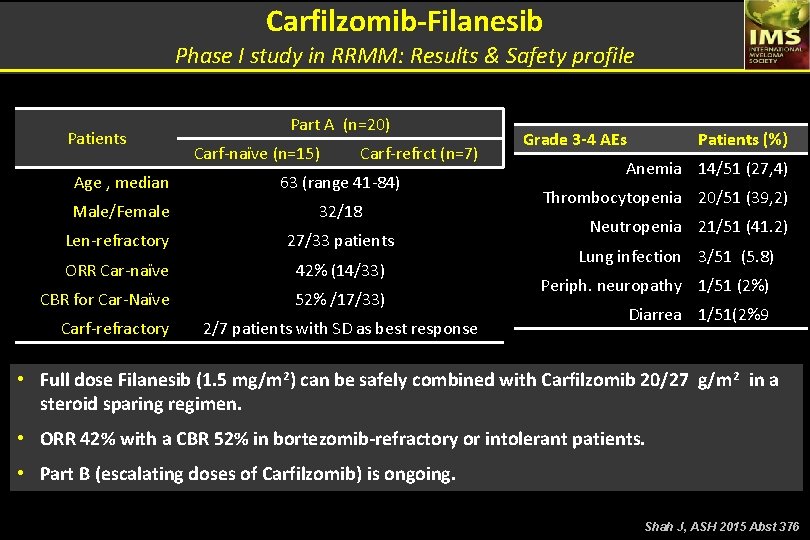
Carfilzomib-Filanesib Phase I study in RRMM: Results & Safety profile Patients Part A (n=20) Carf-naïve (n=15) Carf-refrct (n=7) Age , median 63 (range 41 -84) Male/Female 32/18 Len-refractory 27/33 patients ORR Car-naïve 42% (14/33) CBR for Car-Naïve 52% /17/33) Carf-refractory 2/7 patients with SD as best response Grade 3 -4 AEs Patients (%) Anemia 14/51 (27, 4) Thrombocytopenia 20/51 (39, 2) Neutropenia 21/51 (41. 2) Lung infection 3/51 (5. 8) Periph. neuropathy 1/51 (2%) Diarrea 1/51(2%9 • Full dose Filanesib (1. 5 mg/m 2) can be safely combined with Carfilzomib 20/27 g/m 2 in a steroid sparing regimen. • ORR 42% with a CBR 52% in bortezomib-refractory or intolerant patients. • Part B (escalating doses of Carfilzomib) is ongoing. Shah J, ASH 2015 Abst 376

Phase 2 Study of Carfilzomib (CFZ) with or without Filanesib (FIL) in patients with Advanced Multiple Myeloma (MM) Jeffrey A Zonder, Saad Usmani, Emma C Scott, Craig C. Hofmeister, Nikoletta Lendvai, Jesus G. Berdeja, Larry D. Anderson Jr, Parameswaran Hari, Seema Singhai, Gregory Orloff, Michael Craig, Jason Valent, Wes Lee, Lowell Hart, J Hrom, Stephano Tarantolo, Edward Faber, Gary J Schiller, Jennifer Schreiber, Colleen Oliver, Selena A Rush, Brian Tunquist, Mieke Ptaszynski, Noopur S. Raje. Zonder J, ASH 2015 Abst 728

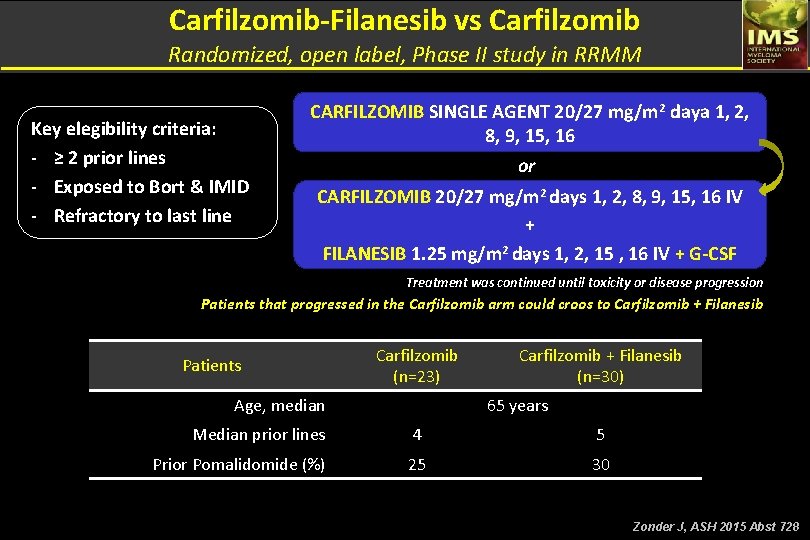
Carfilzomib-Filanesib vs Carfilzomib Randomized, open label, Phase II study in RRMM Key elegibility criteria: - ≥ 2 prior lines - Exposed to Bort & IMID - Refractory to last line CARFILZOMIB SINGLE AGENT 20/27 mg/m 2 daya 1, 2, 8, 9, 15, 16 or CARFILZOMIB 20/27 mg/m 2 days 1, 2, 8, 9, 15, 16 IV + FILANESIB 1. 25 mg/m 2 days 1, 2, 15 , 16 IV + G-CSF Treatment was continued until toxicity or disease progression Patients that progressed in the Carfilzomib arm could croos to Carfilzomib + Filanesib Patients Carfilzomib (n=23) Age, median Carfilzomib + Filanesib (n=30) 65 years Median prior lines 4 5 Prior Pomalidomide (%) 25 30 Zonder J, ASH 2015 Abst 728

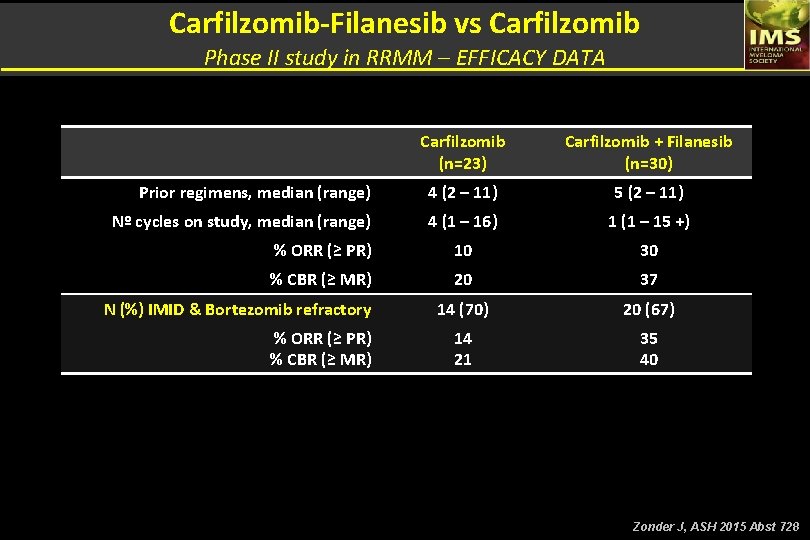
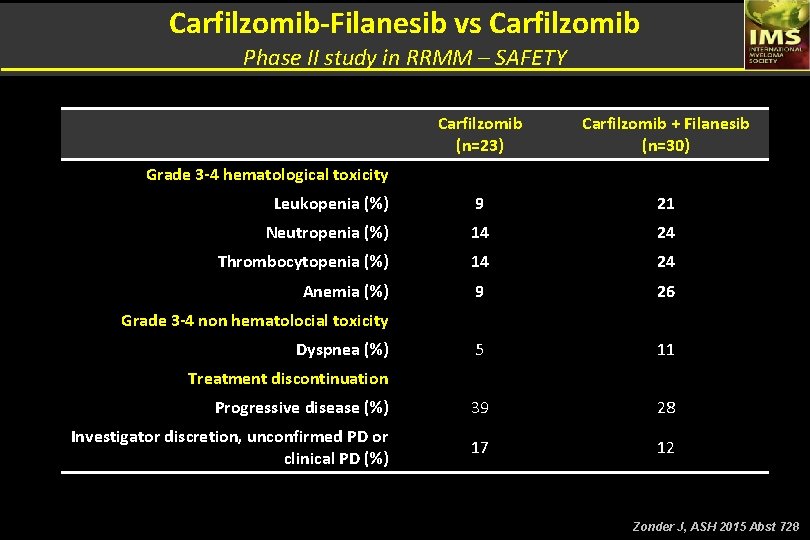
Carfilzomib-Filanesib vs Carfilzomib Phase II study in RRMM – EFFICACY DATA Carfilzomib (n=23) Carfilzomib + Filanesib (n=30) Prior regimens, median (range) 4 (2 – 11) 5 (2 – 11) Nº cycles on study, median (range) 4 (1 – 16) 1 (1 – 15 +) % ORR (≥ PR) 10 30 % CBR (≥ MR) 20 37 14 (70) 20 (67) 14 21 35 40 N (%) IMID & Bortezomib refractory % ORR (≥ PR) % CBR (≥ MR) Zonder J, ASH 2015 Abst 728

Carfilzomib-Filanesib vs Carfilzomib Phase II study in RRMM – SAFETY Carfilzomib (n=23) Carfilzomib + Filanesib (n=30) Leukopenia (%) 9 21 Neutropenia (%) 14 24 Thrombocytopenia (%) 14 24 Anemia (%) 9 26 5 11 Progressive disease (%) 39 28 Investigator discretion, unconfirmed PD or clinical PD (%) 17 12 Grade 3 -4 hematological toxicity Grade 3 -4 non hematolocial toxicity Dyspnea (%) Treatment discontinuation Zonder J, ASH 2015 Abst 728

Combination treatment of the Bruton’s Tyrosine Kinase Inhibitor Ibrutinib and Carfilzomib in Patients with Relapsed or Relapsed and Refractory Multiple Myeloma: Initial Results from a Multicenter Phase 1/2 b Study Ajai Chari, Saurabh Chhabra, Saad Usmani, Sarah Larson, Ruben Niesvizky, Jeffrey Matous, Cristina Gasparetto, Beata Holkova, Matthew Lunning, Jason Valent, Larry D. Anderson Jr. , Chatchada Karanes, Long Kwei, Lipo Chang, Thorsten Graef, Elizabeth Bilotti, Kevin Mc. Donagh. Chari A, ASH 2015 Abst 377

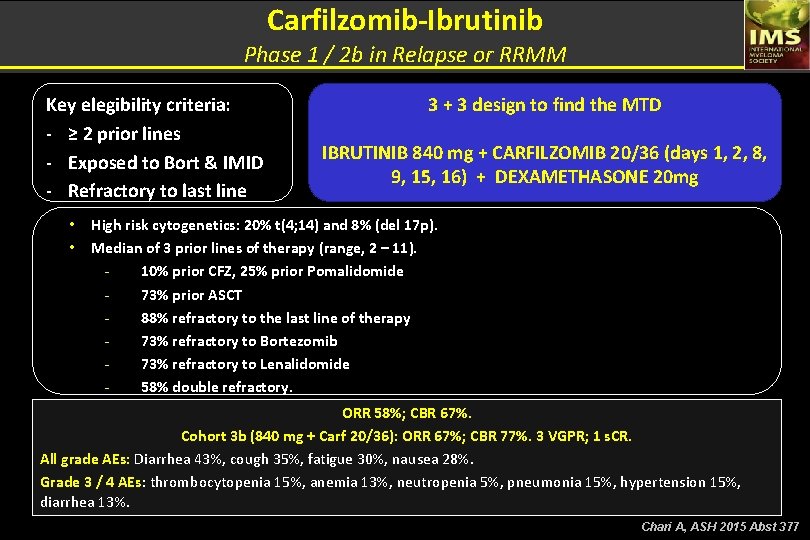
Carfilzomib-Ibrutinib Phase 1 / 2 b in Relapse or RRMM Key elegibility criteria: - ≥ 2 prior lines - Exposed to Bort & IMID - Refractory to last line • • 3 + 3 design to find the MTD IBRUTINIB 840 mg + CARFILZOMIB 20/36 (days 1, 2, 8, 9, 15, 16) + DEXAMETHASONE 20 mg High risk cytogenetics: 20% t(4; 14) and 8% (del 17 p). Median of 3 prior lines of therapy (range, 2 – 11). 10% prior CFZ, 25% prior Pomalidomide 73% prior ASCT 88% refractory to the last line of therapy 73% refractory to Bortezomib 73% refractory to Lenalidomide 58% double refractory. ORR 58%; CBR 67%. Cohort 3 b (840 mg + Carf 20/36): ORR 67%; CBR 77%. 3 VGPR; 1 s. CR. All grade AEs: Diarrhea 43%, cough 35%, fatigue 30%, nausea 28%. Grade 3 / 4 AEs: thrombocytopenia 15%, anemia 13%, neutropenia 5%, pneumonia 15%, hypertension 15%, diarrhea 13%. Chari A, ASH 2015 Abst 377

Alliance A 061202. a Phase I/II Study of Pomalidomide, Dexamethasone and Ixazomib versus Pomalidomide and Dexamethasone for Patients with Multiple Myeloma Refractory to Lenalidomide and Proteasome Inhibitor based Therapy: Phase I Results Peter M. Vorhees, Flora Mulkey, Hani Hassoun, Claudia E. Paba-Paba, Yvonne A. Efebera, Eva Hoke, Guadalupe Aquino, Destin Carlisle, Vera Suman, Paul G. Richardson. Voorhees PM, ASH 2015 Abst 375

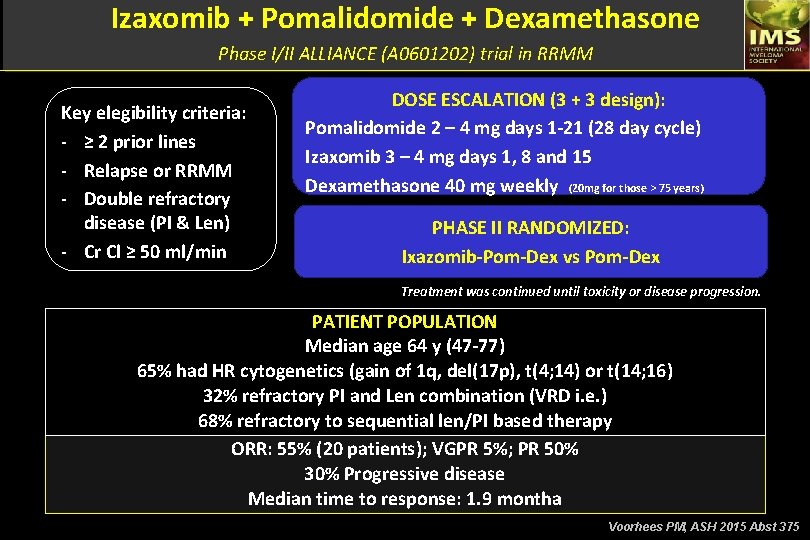
Izaxomib + Pomalidomide + Dexamethasone Phase I/II ALLIANCE (A 0601202) trial in RRMM Key elegibility criteria: - ≥ 2 prior lines - Relapse or RRMM - Double refractory disease (PI & Len) - Cr Cl ≥ 50 ml/min DOSE ESCALATION (3 + 3 design): Pomalidomide 2 – 4 mg days 1 -21 (28 day cycle) Izaxomib 3 – 4 mg days 1, 8 and 15 Dexamethasone 40 mg weekly (20 mg for those > 75 years) PHASE II RANDOMIZED: Ixazomib-Pom-Dex vs Pom-Dex Treatment was continued until toxicity or disease progression. PATIENT POPULATION Median age 64 y (47 -77) 65% had HR cytogenetics (gain of 1 q, del(17 p), t(4; 14) or t(14; 16) 32% refractory PI and Len combination (VRD i. e. ) 68% refractory to sequential len/PI based therapy ORR: 55% (20 patients); VGPR 5%; PR 50% 30% Progressive disease Median time to response: 1. 9 montha Voorhees PM, ASH 2015 Abst 375

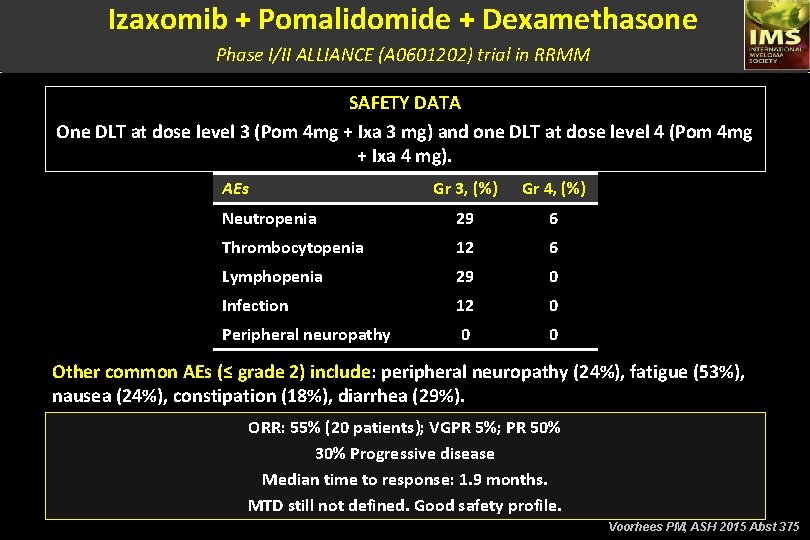
Izaxomib + Pomalidomide + Dexamethasone Phase I/II ALLIANCE (A 0601202) trial in RRMM SAFETY DATA One DLT at dose level 3 (Pom 4 mg + Ixa 3 mg) and one DLT at dose level 4 (Pom 4 mg + Ixa 4 mg). AEs Gr 3, (%) Gr 4, (%) Neutropenia 29 6 Thrombocytopenia 12 6 Lymphopenia 29 0 Infection 12 0 Peripheral neuropathy 0 0 Other common AEs (≤ grade 2) include: peripheral neuropathy (24%), fatigue (53%), nausea (24%), constipation (18%), diarrhea (29%). ORR: 55% (20 patients); VGPR 5%; PR 50% 30% Progressive disease Median time to response: 1. 9 months. MTD still not defined. Good safety profile. Voorhees PM, ASH 2015 Abst 375

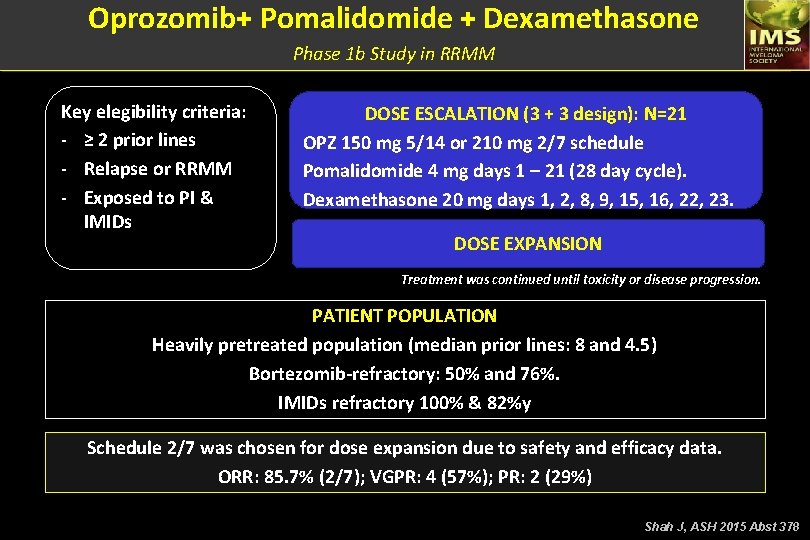
Oprozomib+ Pomalidomide + Dexamethasone Phase 1 b Study in RRMM Key elegibility criteria: - ≥ 2 prior lines - Relapse or RRMM - Exposed to PI & IMIDs DOSE ESCALATION (3 + 3 design): N=21 OPZ 150 mg 5/14 or 210 mg 2/7 schedule Pomalidomide 4 mg days 1 – 21 (28 day cycle). Dexamethasone 20 mg days 1, 2, 8, 9, 15, 16, 22, 23. DOSE EXPANSION Treatment was continued until toxicity or disease progression. PATIENT POPULATION Heavily pretreated population (median prior lines: 8 and 4. 5) Bortezomib-refractory: 50% and 76%. IMIDs refractory 100% & 82%y Schedule 2/7 was chosen for dose expansion due to safety and efficacy data. ORR: 85. 7% (2/7); VGPR: 4 (57%); PR: 2 (29%) Shah J, ASH 2015 Abst 378

Safety of Treatment with Pomalidomide and Low-Dose Dexamethasone in Patients with Relapsed or Refractory Multiple Myeloma and Renal Impairment, Including Those on Dialysis Karthik Ramasamy, 1 Meletios Dimopoulos, 2 Niels Van de Donk, 3 Barbara Gamberi, 4 Frank Bridoux, 5 Matthew Streetly, 6 Elisabeth Kueenburg, 7 Barbara Rosettani, 7 Shona Collins, 7 Frederik Lersch, 7 Pamela Bacon, 7 Katja C. Weisel, 8 Pieter Sonneveld 9 Ramasamy K, ASH 2015 Abst 374

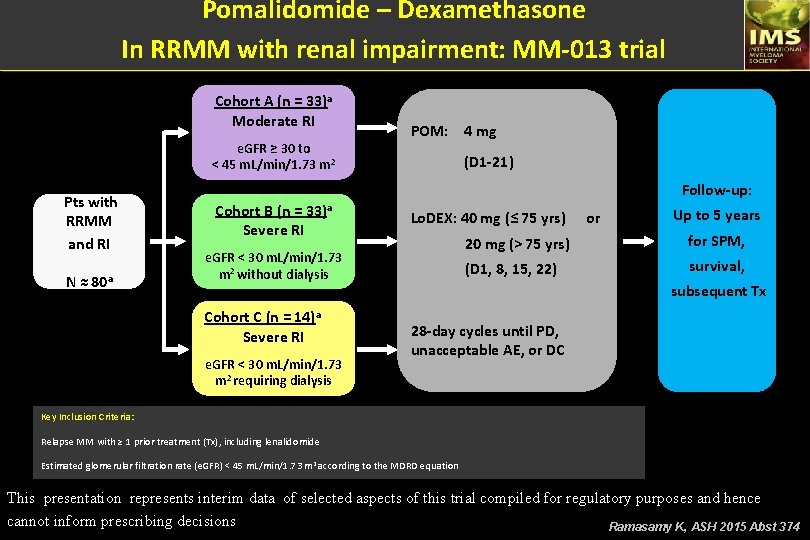
Pomalidomide – Dexamethasone In RRMM with renal impairment: MM-013 trial Cohort A (n = 33)a Moderate RI e. GFR ≥ 30 to < 45 m. L/min/1. 73 m 2 Pts with RRMM and RI N ≈ 80 a POM: 4 mg (D 1 -21) Follow-up: Cohort B (n = 33)a Severe RI e. GFR < 30 m. L/min/1. 73 m 2 without dialysis Cohort C (n = 14)a Severe RI e. GFR < 30 m. L/min/1. 73 m 2 requiring dialysis Lo. DEX: 40 mg (≤ 75 yrs) or Up to 5 years 20 mg (> 75 yrs) for SPM, (D 1, 8, 15, 22) survival, subsequent Tx 28 -day cycles until PD, unacceptable AE, or DC Key Inclusion Criteria: Relapse MM with ≥ 1 prior treatment (Tx), including lenalidomide Estimated glomerular filtration rate (e. GFR) < 45 m. L/min/1. 73 m 2 according to the MDRD equation This presentation represents interim data of selected aspects of this trial compiled for regulatory purposes and hence cannot inform prescribing decisions Ramasamy K, ASH 2015 Abst 374

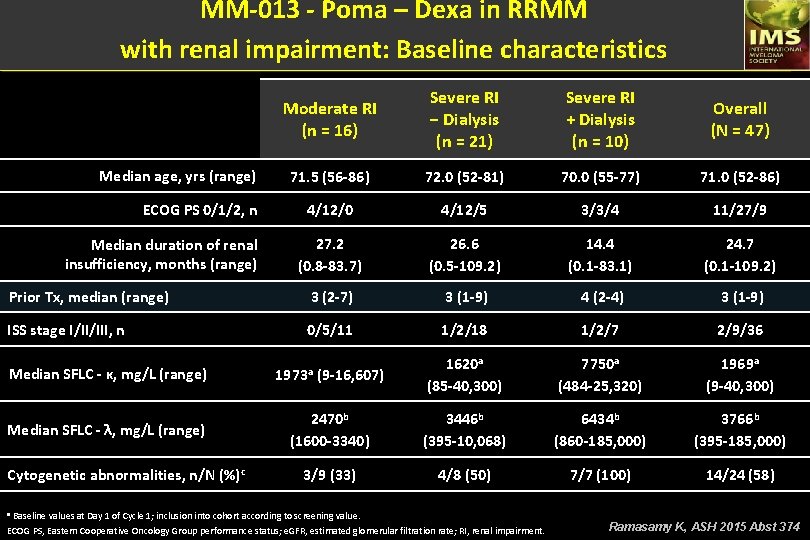
MM-013 - Poma – Dexa in RRMM with renal impairment: Baseline characteristics Moderate RI (n = 16) Severe RI − Dialysis (n = 21) Severe RI + Dialysis (n = 10) Overall (N = 47) 71. 5 (56 -86) 72. 0 (52 -81) 70. 0 (55 -77) 71. 0 (52 -86) 4/12/0 4/12/5 3/3/4 11/27/9 27. 2 (0. 8 -83. 7) 26. 6 (0. 5 -109. 2) 14. 4 (0. 1 -83. 1) 24. 7 (0. 1 -109. 2) Prior Tx, median (range) 3 (2 -7) 3 (1 -9) 4 (2 -4) 3 (1 -9) ISS stage I/II/III, n 0/5/11 1/2/18 1/2/7 2/9/36 Median SFLC - κ, mg/L (range) 1973 a (9 -16, 607) 1620 a (85 -40, 300) 7750 a (484 -25, 320) 1969 a (9 -40, 300) Median SFLC - λ, mg/L (range) 2470 b (1600 -3340) 3446 b (395 -10, 068) 6434 b (860 -185, 000) 3766 b (395 -185, 000) 3/9 (33) 4/8 (50) 7/7 (100) 14/24 (58) Median age, yrs (range) ECOG PS 0/1/2, n Median duration of renal insufficiency, months (range) Cytogenetic abnormalities, n/N (%)c a Baseline values at Day 1 of Cycle 1; inclusion into cohort according to screening value. ECOG PS, Eastern Cooperative Oncology Group performance status; e. GFR, estimated glomerular filtration rate; RI, renal impairment. Ramasamy K, ASH 2015 Abst 374

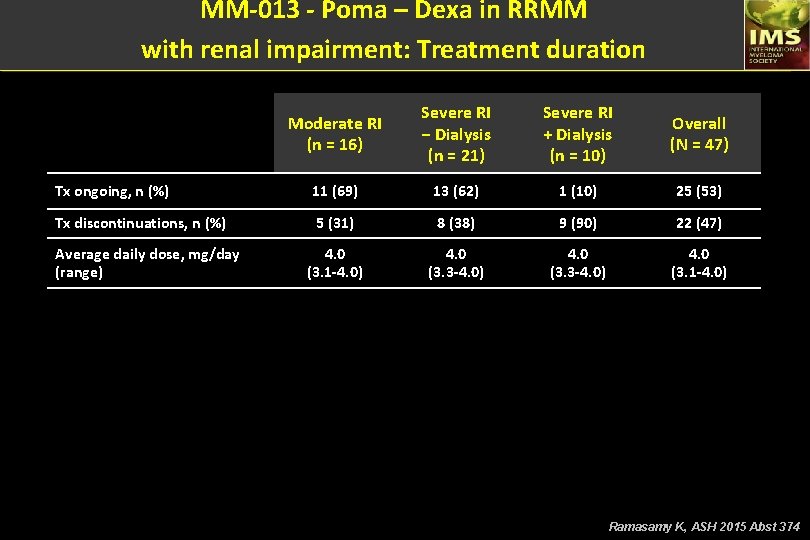
MM-013 - Poma – Dexa in RRMM with renal impairment: Treatment duration Moderate RI (n = 16) Severe RI − Dialysis (n = 21) Severe RI + Dialysis (n = 10) Overall (N = 47) Tx ongoing, n (%) 11 (69) 13 (62) 1 (10) 25 (53) Tx discontinuations, n (%) 5 (31) 8 (38) 9 (90) 22 (47) 4. 0 (3. 1 -4. 0) 4. 0 (3. 3 -4. 0) 4. 0 (3. 1 -4. 0) Average daily dose, mg/day (range) Ramasamy K, ASH 2015 Abst 374

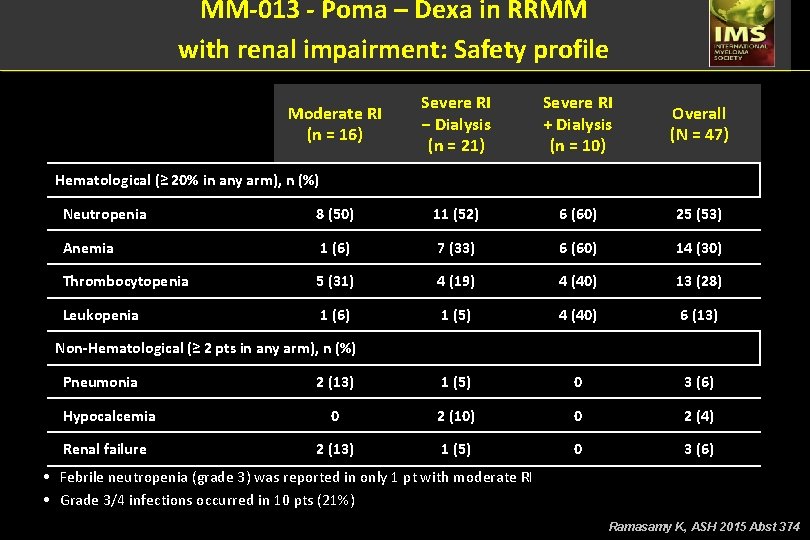
MM-013 - Poma – Dexa in RRMM with renal impairment: Safety profile Moderate RI (n = 16) Severe RI − Dialysis (n = 21) Severe RI + Dialysis (n = 10) Overall (N = 47) Hematological (≥ 20% in any arm), n (%) Neutropenia 8 (50) 11 (52) 6 (60) 25 (53) Anemia 1 (6) 7 (33) 6 (60) 14 (30) Thrombocytopenia 5 (31) 4 (19) 4 (40) 13 (28) Leukopenia 1 (6) 1 (5) 4 (40) 6 (13) 2 (13) 1 (5) 0 3 (6) Hypocalcemia 0 2 (10) 0 2 (4) Renal failure 2 (13) 1 (5) 0 3 (6) Non-Hematological (≥ 2 pts in any arm), n (%) Pneumonia • Febrile neutropenia (grade 3) was reported in only 1 pt with moderate RI • Grade 3/4 infections occurred in 10 pts (21%) Ramasamy K, ASH 2015 Abst 374

MM-013 - Poma – Dexa in RRMM with renal impairment: Conclusions • Overall safety profile in pts with RI was consistent with previously reported safety profile of POM (ie, mainly hematologic AEs events and infections)1 – As expected, the relative number of some AE’s in pts with severe RI requiring dialysis appears higher – Preliminary data suggest no significant POM exposure differences among RI cohorts – Median relative dose intensity was ≥ 0. 97 in all pt cohorts • The MM-013 study is ongoing AE, adverse event; Lo. DEX, low-dose dexamethasone; POM, pomalidomide; pt, patient; RI, renal impairment. 1. San Miguel J, et al. Lancet Oncol. 2013; 14: 1055 -1066. Ramasamy K, ASH 2015 Abst 374