Raman Scattering Tyndall scattering if small particles are



- Slides: 19

Raman

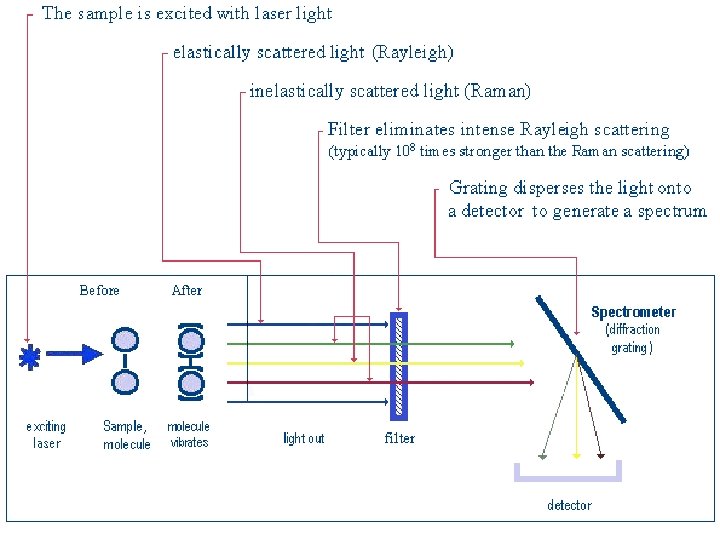
Scattering • Tyndall scattering – if small particles are present • During Rayleigh scattering (interaction of light with relatively small molecules) incident light is scattered in all directions • Some of the incident energy can be converted into rotational or vibrational energy- so wavelength of scattered light is longer

In a fluorescence experiment, the scattered light will be collected along with the fluorescence • Thus we may see peaks in our ‘fluorescence’ spectrum that do not arise by emission. • Especially true with low levels of fluorescence

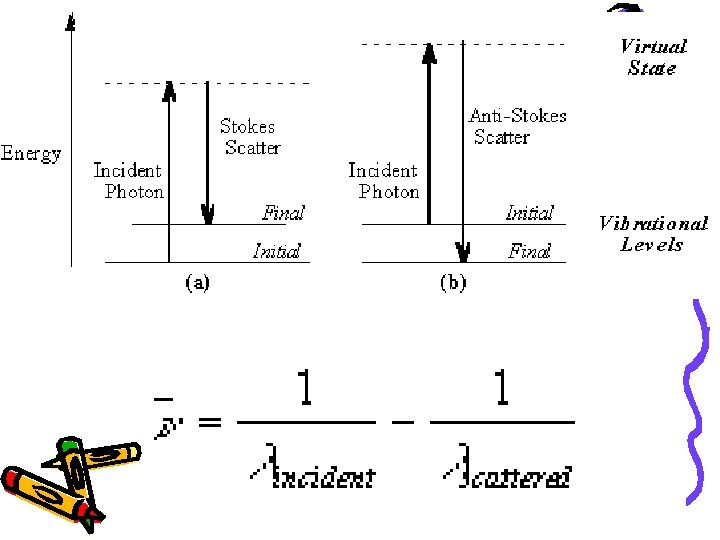
• The Raman effect arises when a photon is incident on a molecule and interacts with the electric dipole and causes perturbation • In quantum mechanics the scattering is described as an excitation to a virtual state lower in energy than a real electronic transition with nearly coincident de-excitation and a change in vibrational energy.



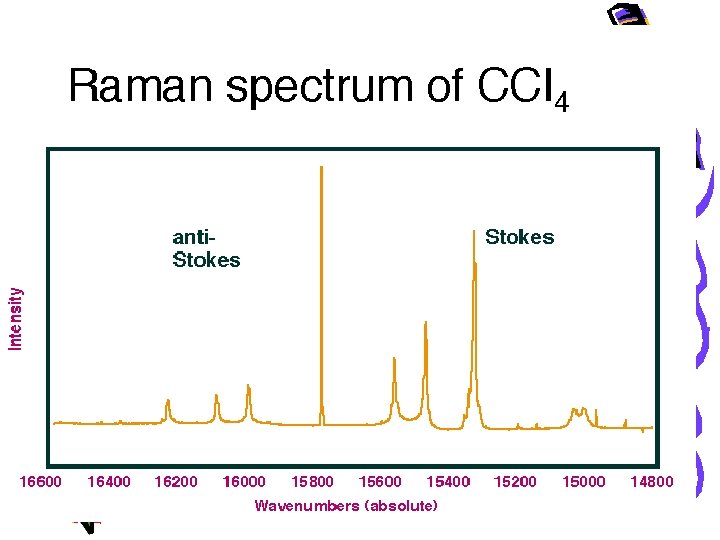
• At room temperature thermal population of vibrational excited states is low • Therefore the initial state is usually the ground state and the scattered photon will have lower energy (longer wavelength) than the exciting photon. • This Stokes shift is what is usually observed in Raman spectroscopy.

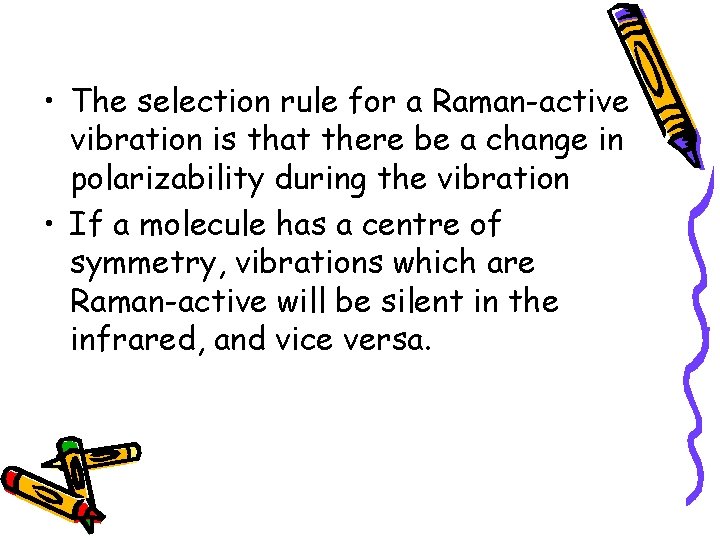
• The selection rule for a Raman-active vibration is that there be a change in polarizability during the vibration • If a molecule has a centre of symmetry, vibrations which are Raman-active will be silent in the infrared, and vice versa.


Polarization effects • Raman scatter from totally symmetric vibrations will be strongly polarized parallel to the plane of polarization of the incident light. • The scattered intensity from nontotally symmetric vibrations is ¾ as strong in the plane perpendicular to the plane of polarization of the incident light as in the plane parallel to it.

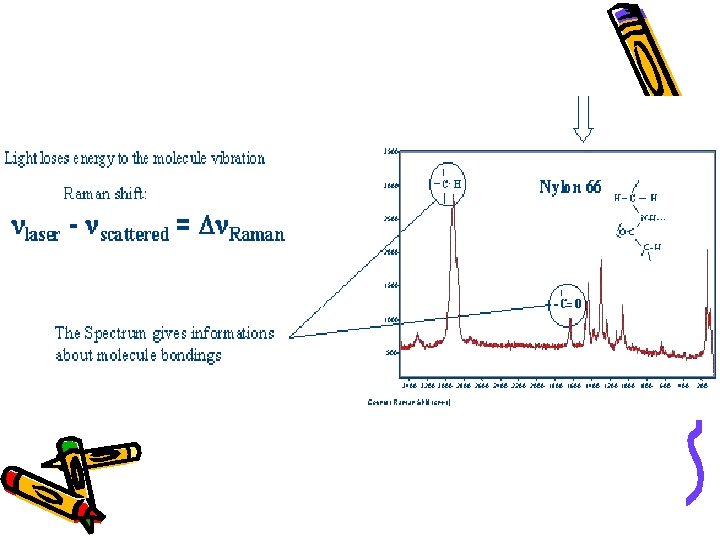
• Typical strong Raman scatterers are moieties with distributed electron clouds, such as carbon-carbon double bonds. • The pi-electron cloud of the double bond is easily distorted in an external electric field. • Bending or stretching the bond changes the distribution of electron density substantially, and causes a large change in induced dipole moment.

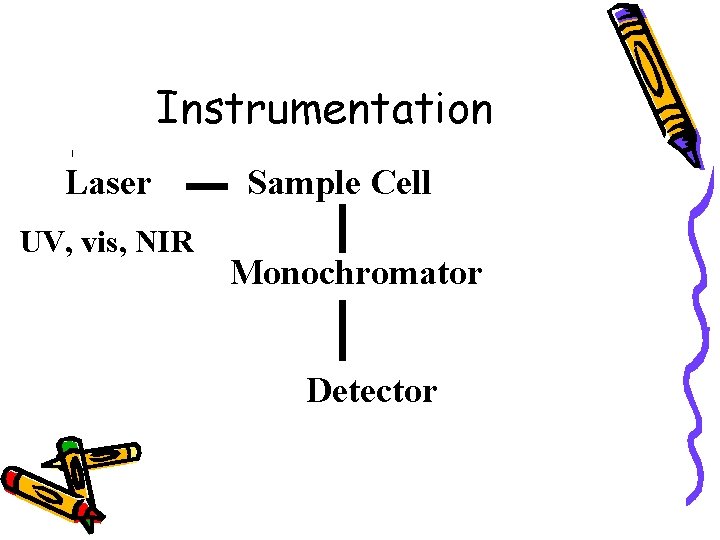
Instrumentation Laser UV, vis, NIR Sample Cell Monochromator Detector


Fluorescence Interferes • Just as a Raman peak can show up in a fluorescence spectrum, fluorescence can show up in – and often swamps out – a Raman spectrum • Change laser wavelength – to longer wavelength • Raman shifts are independent of the wavelength of excitation

In summary: • Raman spectroscopy gives information about vibrations • It uses UV, visible or NIR laser light rather than IR • The information is often complementary to that obtaine by IR – especially for molecules with a centre of symmetry • Water is less of a problem Can use quartz cells and fibre optics

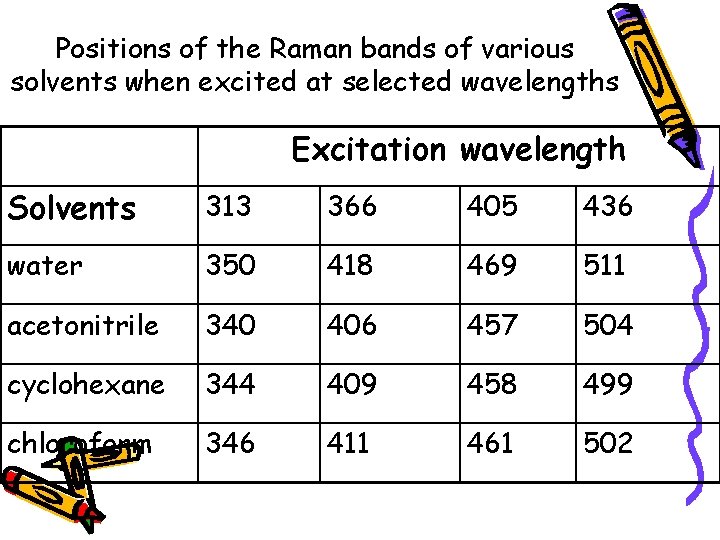
Positions of the Raman bands of various solvents when excited at selected wavelengths Excitation wavelength Solvents 313 366 405 436 water 350 418 469 511 acetonitrile 340 406 457 504 cyclohexane 344 409 458 499 chloroform 346 411 461 502

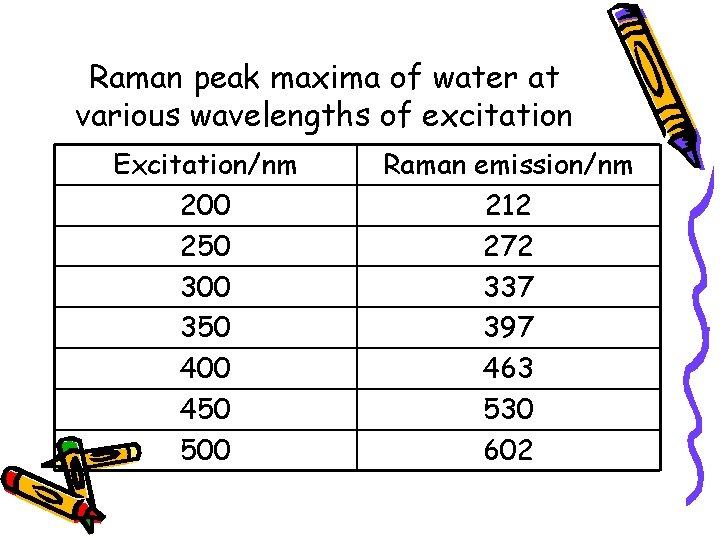
Raman peak maxima of water at various wavelengths of excitation Excitation/nm 200 250 300 350 400 450 500 Raman emission/nm 212 272 337 397 463 530 602

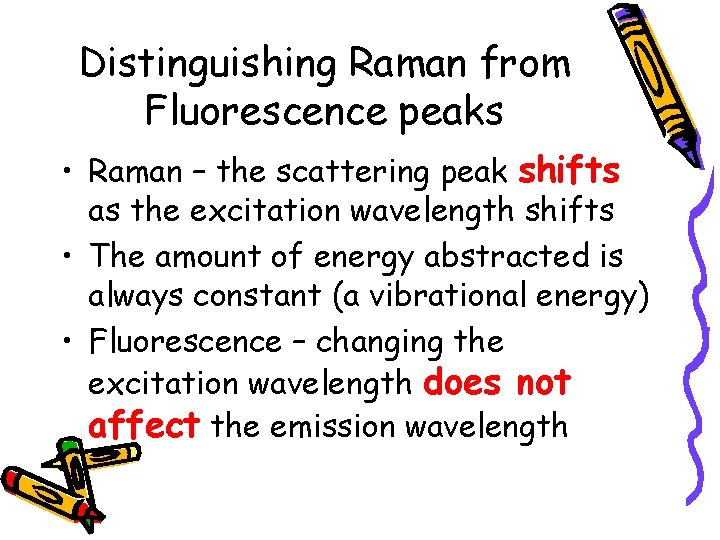
Distinguishing Raman from Fluorescence peaks • Raman – the scattering peak shifts as the excitation wavelength shifts • The amount of energy abstracted is always constant (a vibrational energy) • Fluorescence – changing the excitation wavelength does not affect the emission wavelength

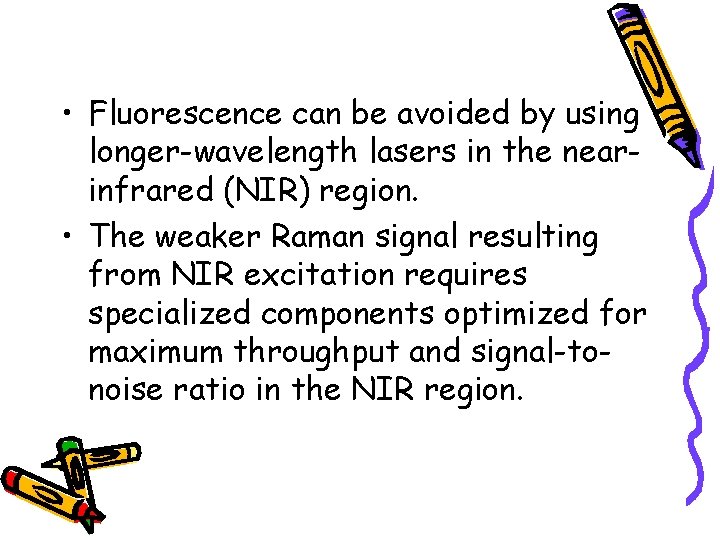
• Fluorescence can be avoided by using longer-wavelength lasers in the nearinfrared (NIR) region. • The weaker Raman signal resulting from NIR excitation requires specialized components optimized for maximum throughput and signal-tonoise ratio in the NIR region.