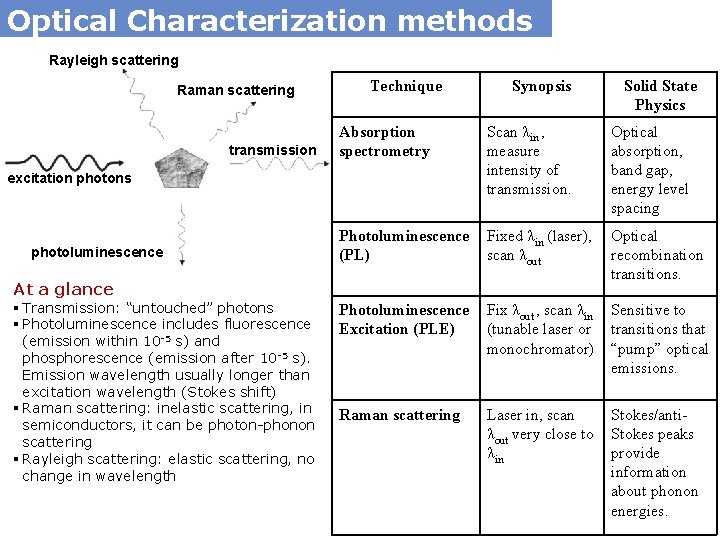
Optical Characterization methods Rayleigh scattering Raman scattering transmission
- Slides: 6

Optical Characterization methods Rayleigh scattering Raman scattering transmission Technique Scan λin , measure intensity of transmission. Optical absorption, band gap, energy level spacing Photoluminescence (PL) Fixed λin (laser), scan λout Optical recombination transitions. Photoluminescence Excitation (PLE) Fix λout , scan λin (tunable laser or monochromator) Sensitive to transitions that “pump” optical emissions. Raman scattering Laser in, scan λout very close to λin Stokes/anti. Stokes peaks provide information about phonon energies. At a glance § Transmission: “untouched” photons § Photoluminescence includes fluorescence (emission within 10 -5 s) and phosphorescence (emission after 10 -5 s). Emission wavelength usually longer than excitation wavelength (Stokes shift) § Raman scattering: inelastic scattering, in semiconductors, it can be photon-phonon scattering § Rayleigh scattering: elastic scattering, no change in wavelength Solid State Physics Absorption spectrometry excitation photons photoluminescence Synopsis

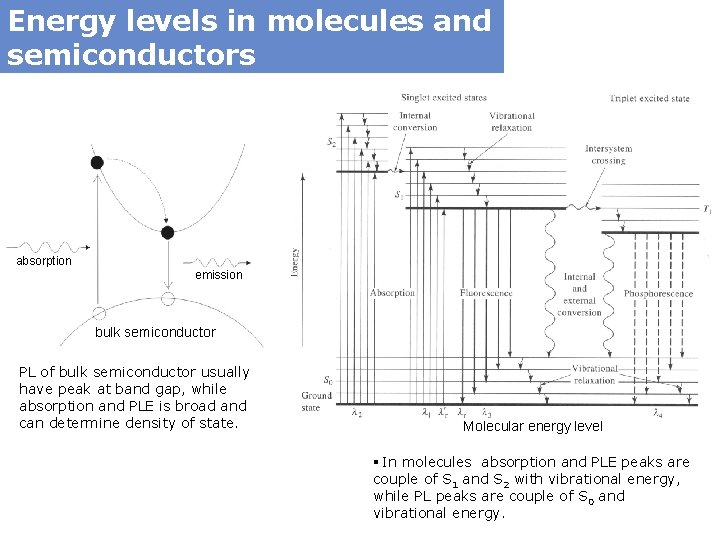
Energy levels in molecules and semiconductors absorption emission bulk semiconductor PL of bulk semiconductor usually have peak at band gap, while absorption and PLE is broad and can determine density of state. Molecular energy level § In molecules absorption and PLE peaks are couple of S 1 and S 2 with vibrational energy, while PL peaks are couple of S 0 and vibrational energy.

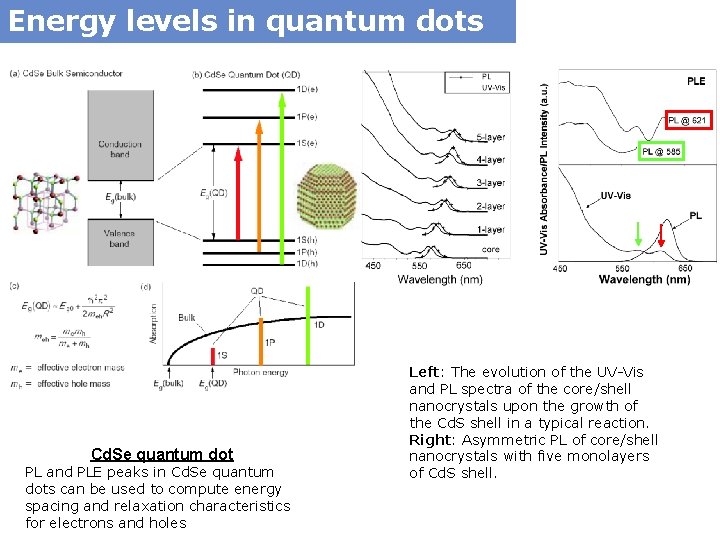
Energy levels in quantum dots Cd. Se quantum dot PL and PLE peaks in Cd. Se quantum dots can be used to compute energy spacing and relaxation characteristics for electrons and holes Left: The evolution of the UV-Vis and PL spectra of the core/shell nanocrystals upon the growth of the Cd. S shell in a typical reaction. Right: Asymmetric PL of core/shell nanocrystals with five monolayers of Cd. S shell.

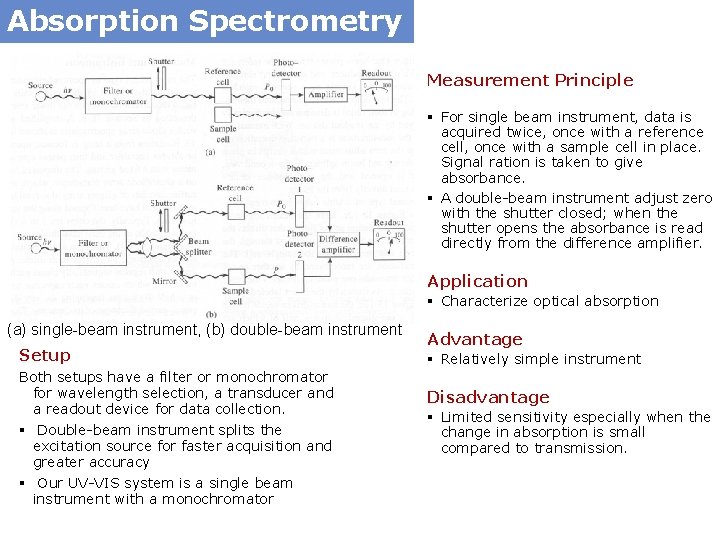
Absorption Spectrometry Measurement Principle § For single beam instrument, data is acquired twice, once with a reference cell, once with a sample cell in place. Signal ration is taken to give absorbance. § A double-beam instrument adjust zero with the shutter closed; when the shutter opens the absorbance is read directly from the difference amplifier. Application § Characterize optical absorption (a) single-beam instrument, (b) double-beam instrument Setup Both setups have a filter or monochromator for wavelength selection, a transducer and a readout device for data collection. § Double-beam instrument splits the excitation source for faster acquisition and greater accuracy § Our UV-VIS system is a single beam instrument with a monochromator Advantage § Relatively simple instrument Disadvantage § Limited sensitivity especially when the change in absorption is small compared to transmission.

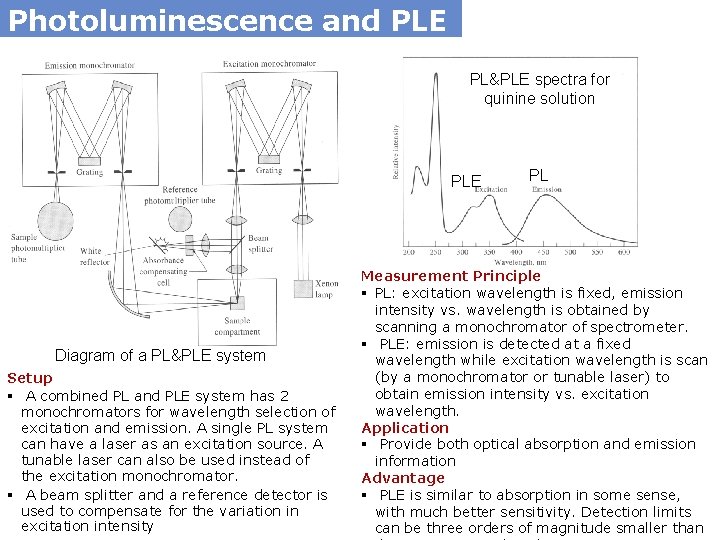
Photoluminescence and PLE PL&PLE spectra for quinine solution PLE Diagram of a PL&PLE system Setup § A combined PL and PLE system has 2 monochromators for wavelength selection of excitation and emission. A single PL system can have a laser as an excitation source. A tunable laser can also be used instead of the excitation monochromator. § A beam splitter and a reference detector is used to compensate for the variation in excitation intensity PL Measurement Principle § PL: excitation wavelength is fixed, emission intensity vs. wavelength is obtained by scanning a monochromator of spectrometer. § PLE: emission is detected at a fixed wavelength while excitation wavelength is scan (by a monochromator or tunable laser) to obtain emission intensity vs. excitation wavelength. Application § Provide both optical absorption and emission information Advantage § PLE is similar to absorption in some sense, with much better sensitivity. Detection limits can be three orders of magnitude smaller than

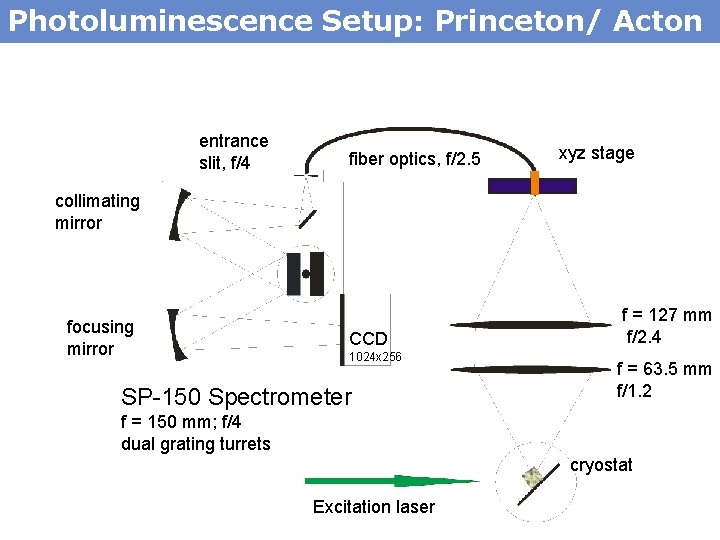
Photoluminescence Setup: Princeton/ Acton entrance slit, f/4 fiber optics, f/2. 5 xyz stage collimating mirror focusing mirror CCD 1024 x 256 SP-150 Spectrometer f = 127 mm f/2. 4 f = 63. 5 mm f/1. 2 f = 150 mm; f/4 dual grating turrets cryostat Excitation laser