quantitative phase analysis Rietveld refinement quantitative phase analysis







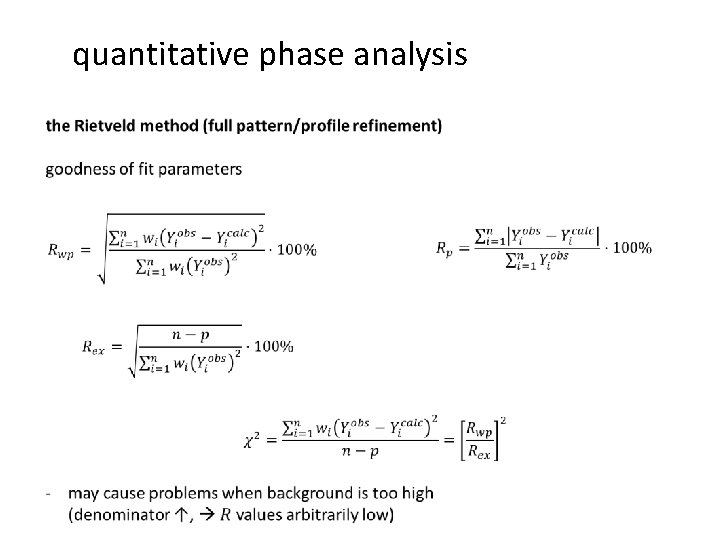


- Slides: 43

quantitative phase analysis + Rietveld refinement

quantitative phase analysis determination of the volume/weight fractions of known crystalline phases in a sample - in order to arrive at plausible results, one should take care of - alignment of the diffractometer - application of suitable optical elements - calibration of the instrumental function - calibration of the absorption coefficients of different phases - sample preparation method - sample-induced effects cannot be eliminated at all times ( minimise, calculate) - preferred orientations - absorption

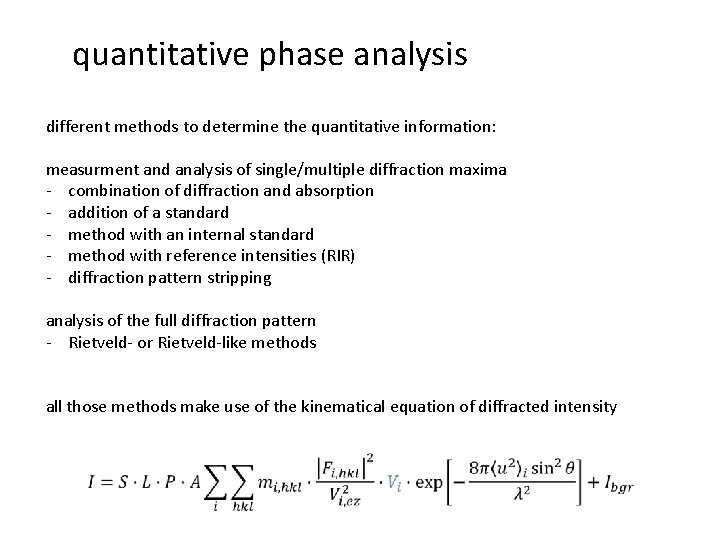
quantitative phase analysis different methods to determine the quantitative information: measurment and analysis of single/multiple diffraction maxima - combination of diffraction and absorption - addition of a standard - method with an internal standard - method with reference intensities (RIR) - diffraction pattern stripping analysis of the full diffraction pattern - Rietveld- or Rietveld-like methods

quantitative phase analysis different methods to determine the quantitative information: measurment and analysis of single/multiple diffraction maxima - combination of diffraction and absorption - addition of a standard - method with an internal standard - method with reference intensities (RIR) - diffraction pattern stripping analysis of the full diffraction pattern - Rietveld- or Rietveld-like methods all those methods make use of the kinematical equation of diffracted intensity

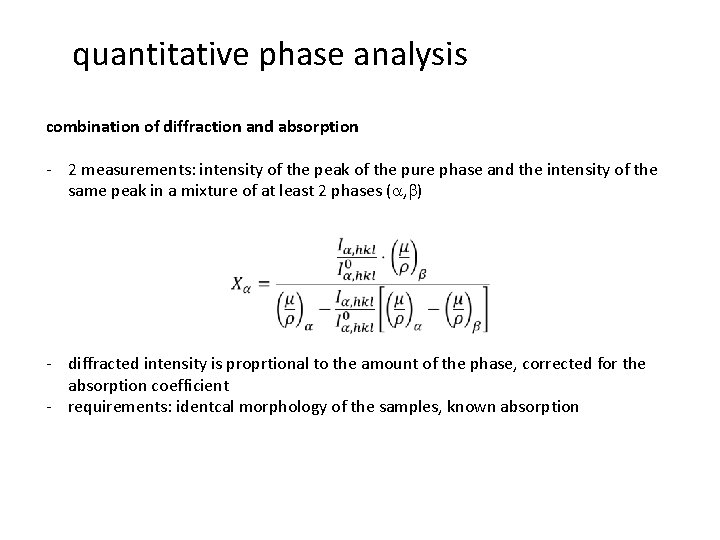
quantitative phase analysis combination of diffraction and absorption - 2 measurements: intensity of the peak of the pure phase and the intensity of the same peak in a mixture of at least 2 phases (a, b) - diffracted intensity is proprtional to the amount of the phase, corrected for the absorption coefficient - requirements: identcal morphology of the samples, known absorption

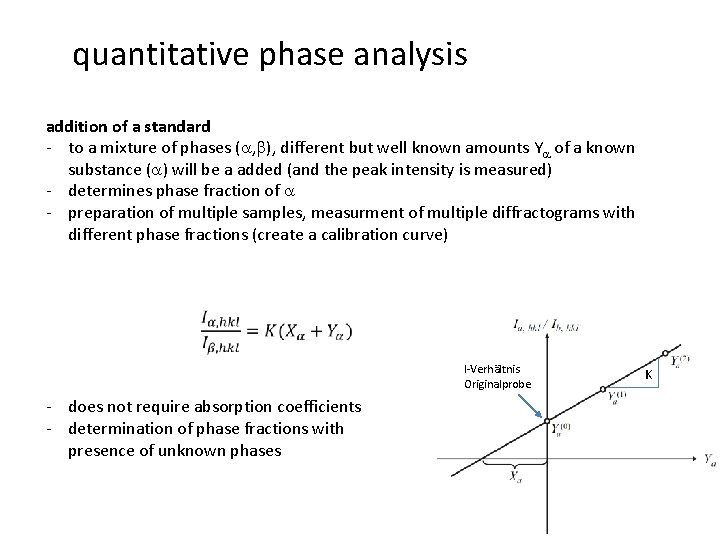
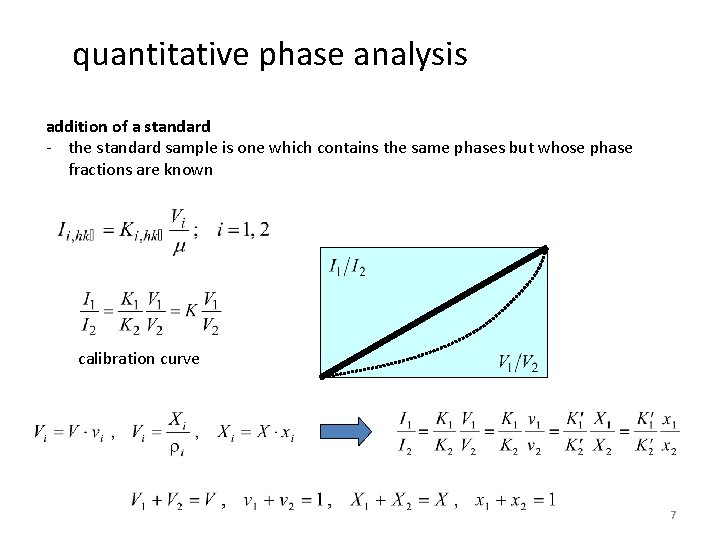
quantitative phase analysis addition of a standard - to a mixture of phases (a, b), different but well known amounts Ya of a known substance (a) will be a added (and the peak intensity is measured) - determines phase fraction of a - preparation of multiple samples, measurment of multiple diffractograms with different phase fractions (create a calibration curve) I-Verhältnis Originalprobe - does not require absorption coefficients - determination of phase fractions with presence of unknown phases K

quantitative phase analysis addition of a standard - the standard sample is one which contains the same phases but whose phase fractions are known calibration curve 7

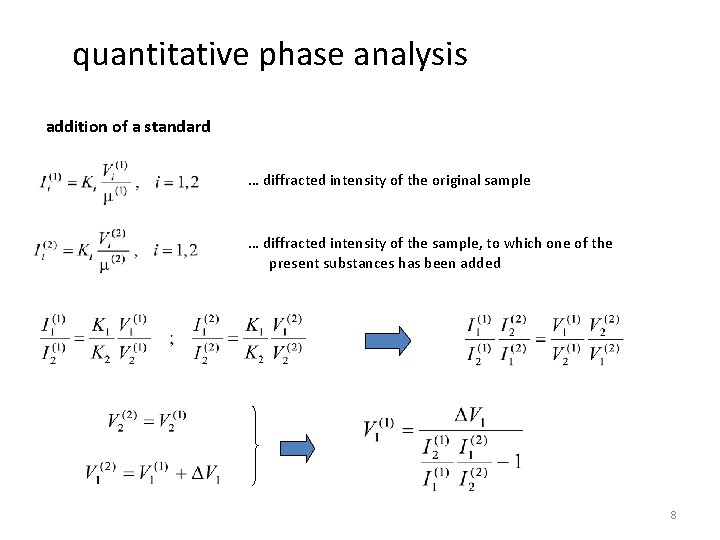
quantitative phase analysis addition of a standard … diffracted intensity of the original sample … diffracted intensity of the sample, to which one of the present substances has been added 8

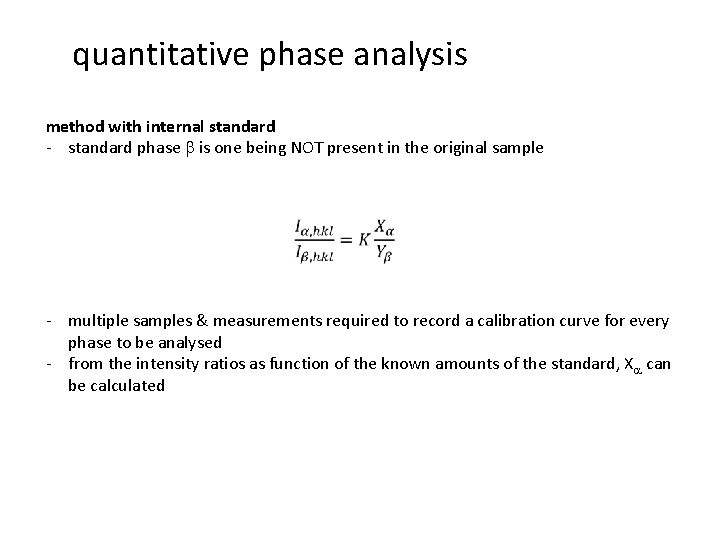
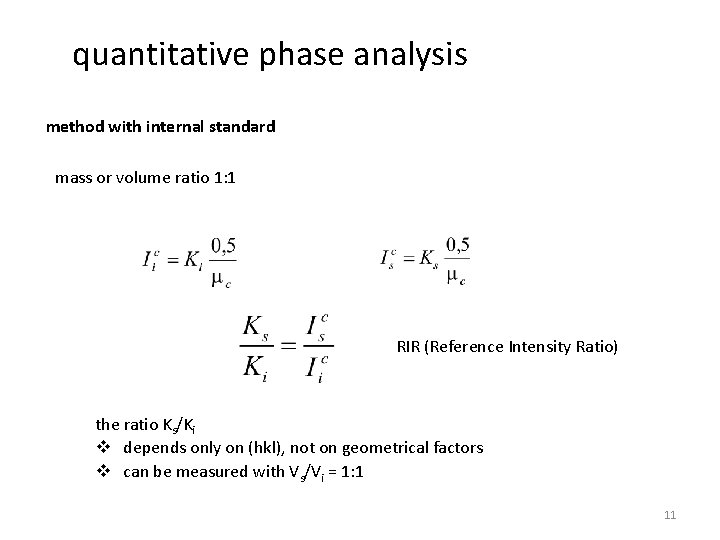
quantitative phase analysis method with internal standard - standard phase b is one being NOT present in the original sample - multiple samples & measurements required to record a calibration curve for every phase to be analysed - from the intensity ratios as function of the known amounts of the standard, Xa can be calculated

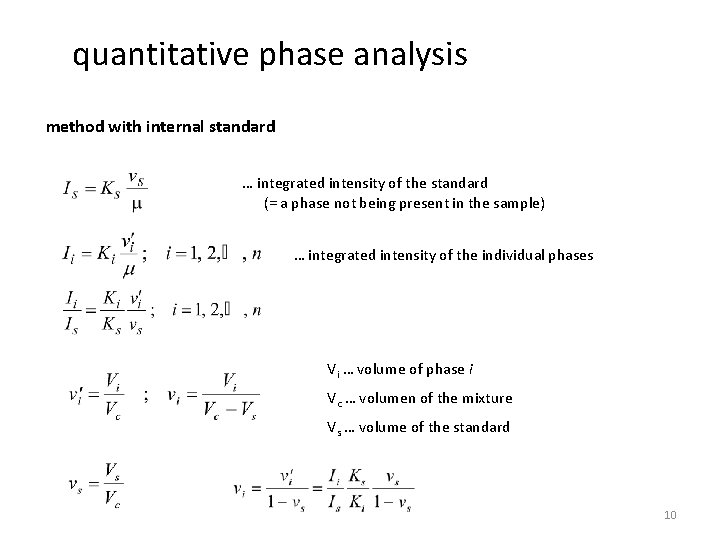
quantitative phase analysis method with internal standard … integrated intensity of the standard (= a phase not being present in the sample) … integrated intensity of the individual phases Vi … volume of phase i Vc … volumen of the mixture Vs … volume of the standard 10

quantitative phase analysis method with internal standard mass or volume ratio 1: 1 RIR (Reference Intensity Ratio) the ratio Ks/Ki v depends only on (hkl), not on geometrical factors v can be measured with Vs/Vi = 1: 1 11

quantitative phase analysis reference intensity method - stärkster Bragg-Peak der betreffenden Phase wird auf den stärksten Peak eines Referenzmaterials (z. B. 113 in Al 2 O 3) bezogen - in ICDD: „corundum number“ = Intensität des stärksten Peaks einer 50: 50 Mischung der Phase mit Korund difficulties: - instrumental parameters/setup - violation of the powder condition

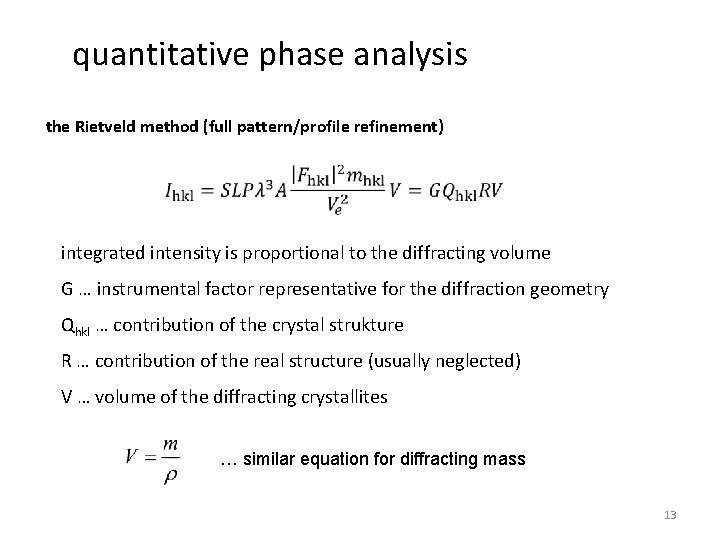
quantitative phase analysis the Rietveld method (full pattern/profile refinement) integrated intensity is proportional to the diffracting volume G … instrumental factor representative for the diffraction geometry Qhkl … contribution of the crystal strukture R … contribution of the real structure (usually neglected) V … volume of the diffracting crystallites … similar equation for diffracting mass 13

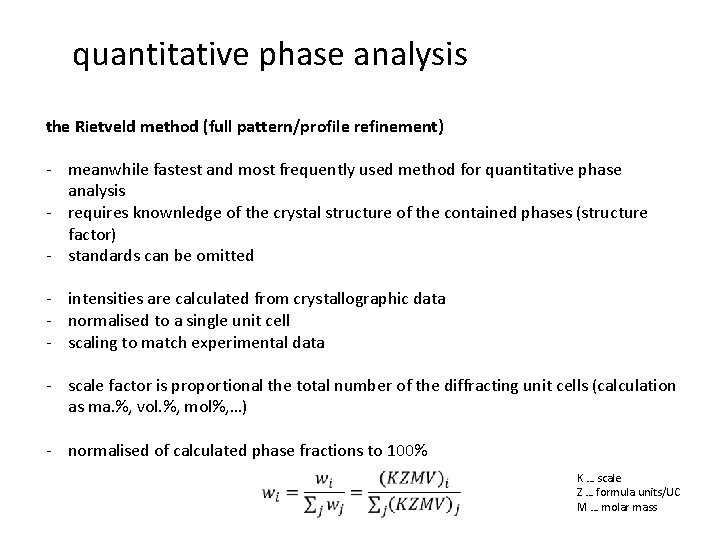
quantitative phase analysis the Rietveld method (full pattern/profile refinement) - meanwhile fastest and most frequently used method for quantitative phase analysis - requires knownledge of the crystal structure of the contained phases (structure factor) - standards can be omitted - intensities are calculated from crystallographic data - normalised to a single unit cell - scaling to match experimental data - scale factor is proportional the total number of the diffracting unit cells (calculation as ma. %, vol. %, mol%, …) - normalised of calculated phase fractions to 100% K … scale Z … formula units/UC M … molar mass

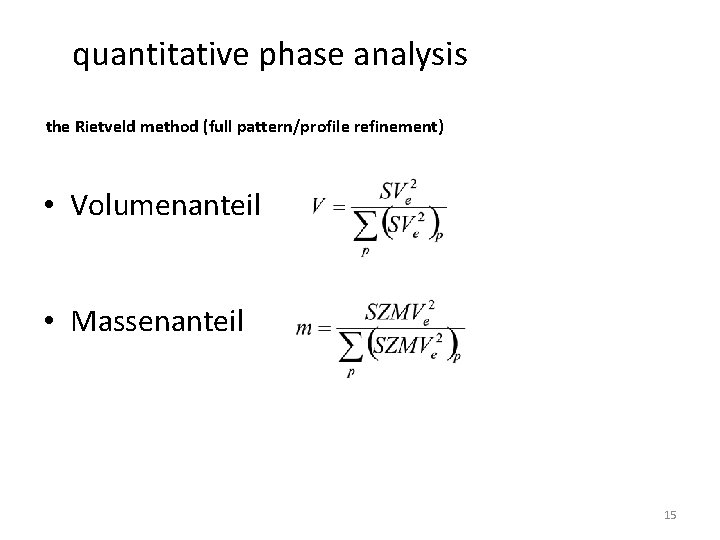
quantitative phase analysis the Rietveld method (full pattern/profile refinement) • Volumenanteil • Massenanteil 15

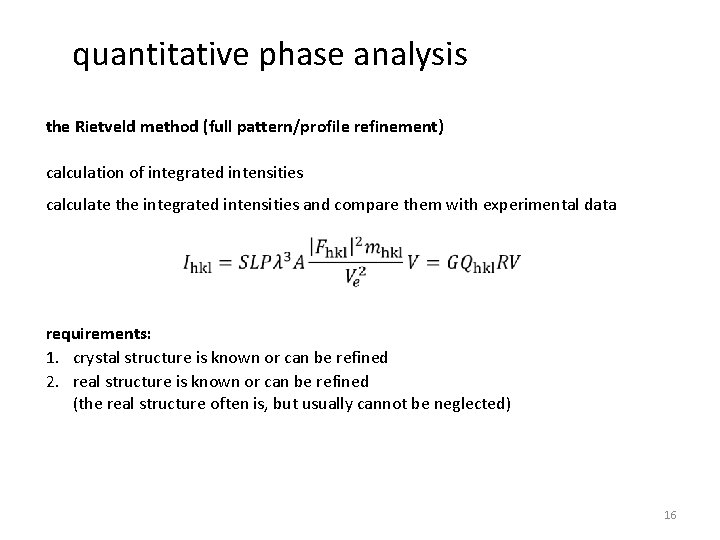
quantitative phase analysis the Rietveld method (full pattern/profile refinement) calculation of integrated intensities calculate the integrated intensities and compare them with experimental data requirements: 1. crystal structure is known or can be refined 2. real structure is known or can be refined (the real structure often is, but usually cannot be neglected) 16

quantitative phase analysis the Rietveld method (full pattern/profile refinement) - requires not only refinement of scale factors (volume fractions) but also of some variables of the structure model - the (real) structure model must be physically/chemically justified - integrated intensities cannot be varied independently, but are governed by the geometry of the structure (structure factor) - refinement using non-linear least squares fitting (NLSF)

quantitative phase analysis the Rietveld method (full pattern/profile refinement) - parameters: - unit cell paramaters - atomic parameters (occupancy, positions, …) - peak shape (-> crystallite size, microstrain) - background - scale factor (-> volume fractions) - Debye-Waller factor (-> thermal motion, point defects, etc. ) - instrumental parameters - search for global minimum via refinement! - refinement strategy determines fit quality

quantitative phase analysis the Rietveld method (full pattern/profile refinement) • analysis of the complete diffraction pattern • refinement of structure and rel structure parameters • NOT suitable for crystal structure solution – the crystal structure must be known a priori (phase, phase composition, lattice parameters, space group, fractional coordinates for every atom in the unit cell) 19

quantitative phase analysis the Rietveld method (full pattern/profile refinement) fixed parameters • space group • chemical composition • analytic function for the line shape • wavelength of X-rays or neutrons • intensity ratio Ka 1/Ka 2 20

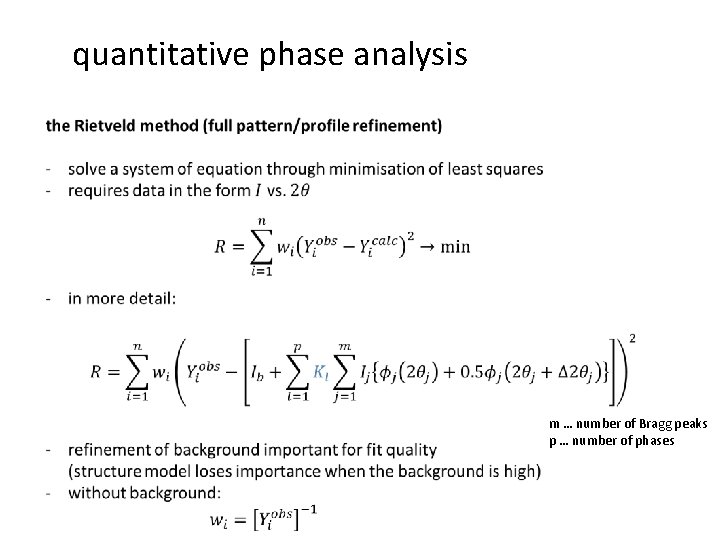
quantitative phase analysis m … number of Bragg peaks p … number of phases

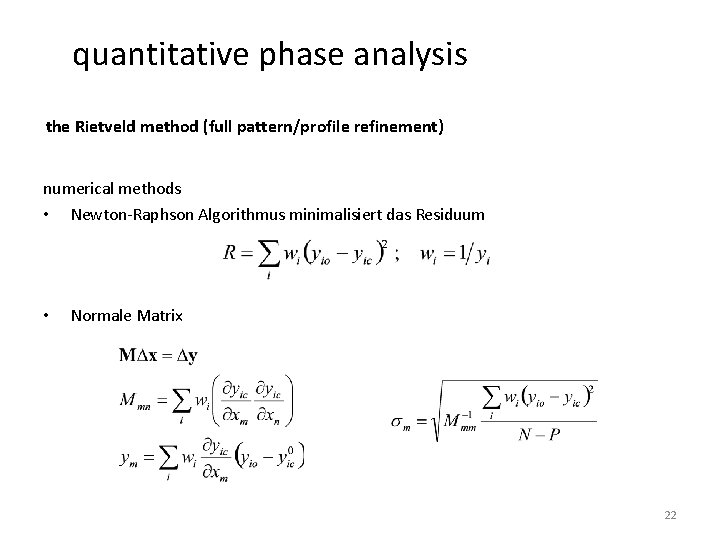
quantitative phase analysis the Rietveld method (full pattern/profile refinement) numerical methods • Newton-Raphson Algorithmus minimalisiert das Residuum • Normale Matrix 22

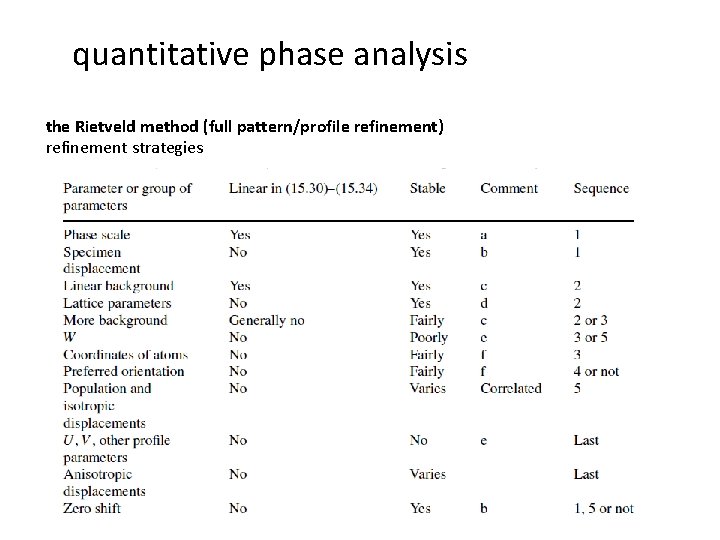
quantitative phase analysis the Rietveld method (full pattern/profile refinement) refinement strategies - background parameters scale factor sample displacement, sample transparency, zero point error peak shape (FWHM, asymmetrie, Gauss/Lorentz) unit cell preferred orientation, absorption, porosity, extinction (phase-dependent) atomic positions, occupancies thermal motion - When and how are parameters refined?

quantitative phase analysis the Rietveld method (full pattern/profile refinement) refinement strategies

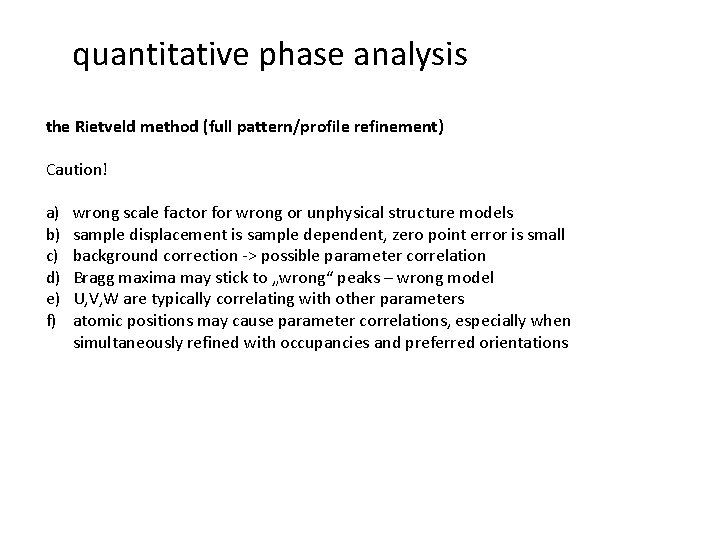
quantitative phase analysis the Rietveld method (full pattern/profile refinement) Caution! a) b) c) d) e) f) wrong scale factor for wrong or unphysical structure models sample displacement is sample dependent, zero point error is small background correction -> possible parameter correlation Bragg maxima may stick to „wrong“ peaks – wrong model U, V, W are typically correlating with other parameters atomic positions may cause parameter correlations, especially when simultaneously refined with occupancies and preferred orientations

quantitative phase analysis the Rietveld method (full pattern/profile refinement) restrictions/conditions - stability of refinement low, when data is of poor quality and complexity of the problem is high - stabilisation of the refinement through constraints (chemically, crystallographically, geometrically, …) - additional information must be correct! - z. B. symmetry of the structure, charge neutrality, substitutional solid solutions, maxima of solubility, …

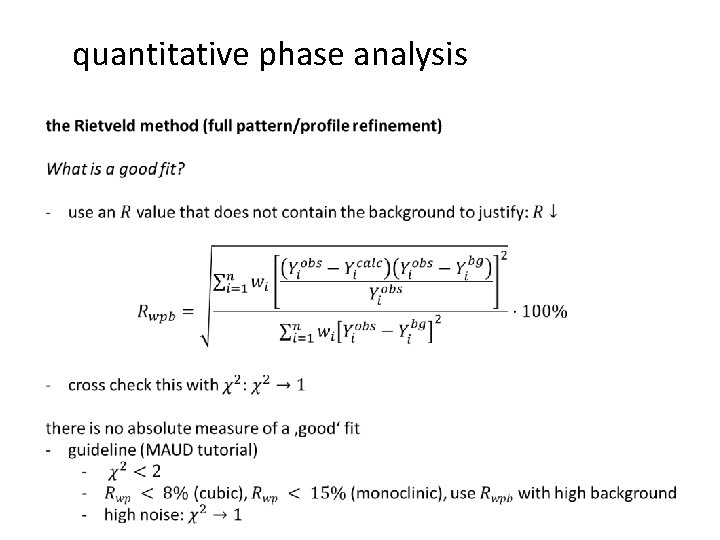
quantitative phase analysis the Rietveld method (full pattern/profile refinement) • 27

quantitative phase analysis

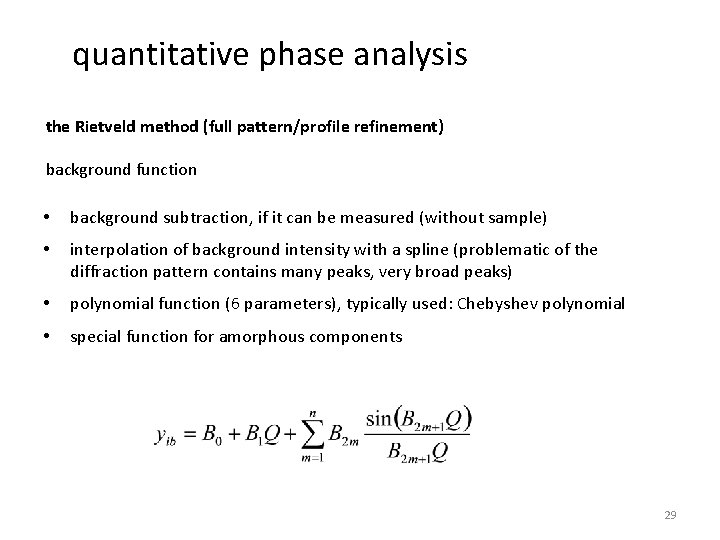
quantitative phase analysis the Rietveld method (full pattern/profile refinement) background function • background subtraction, if it can be measured (without sample) • interpolation of background intensity with a spline (problematic of the diffraction pattern contains many peaks, very broad peaks) • polynomial function (6 parameters), typically used: Chebyshev polynomial • special function for amorphous components 29

quantitative phase analysis

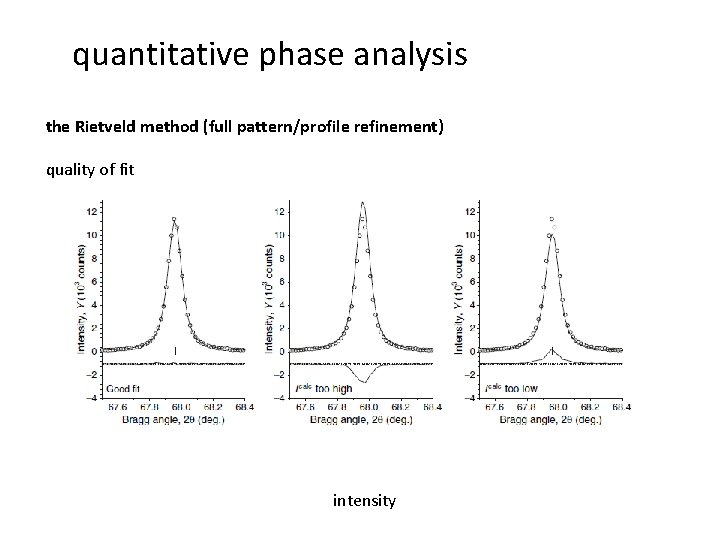
quantitative phase analysis the Rietveld method (full pattern/profile refinement) quality of fit intensity

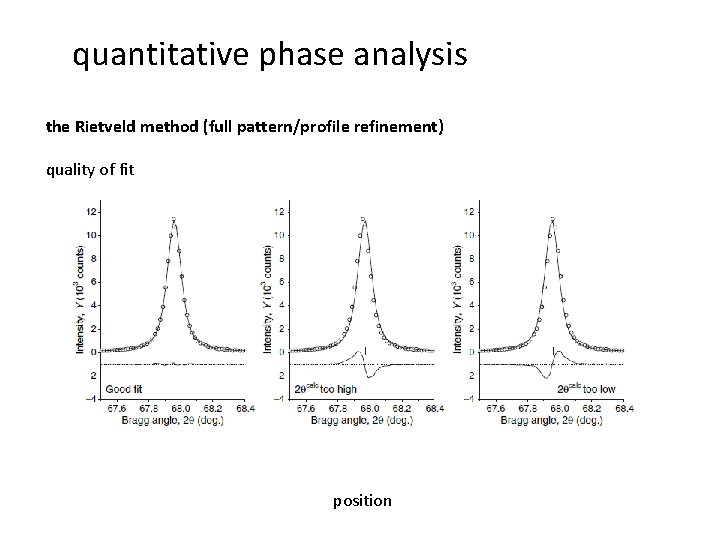
quantitative phase analysis the Rietveld method (full pattern/profile refinement) quality of fit position

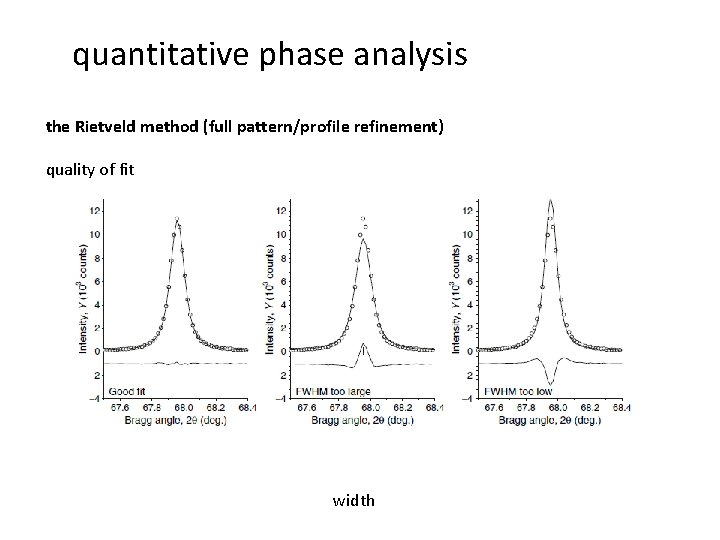
quantitative phase analysis the Rietveld method (full pattern/profile refinement) quality of fit width

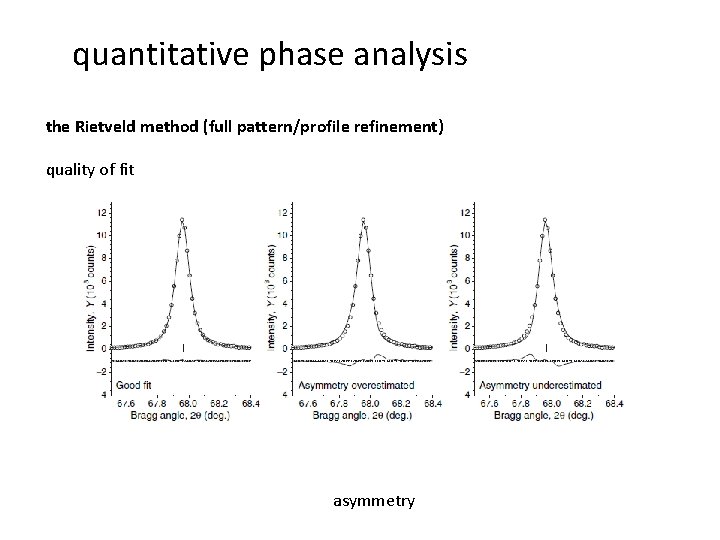
quantitative phase analysis the Rietveld method (full pattern/profile refinement) quality of fit asymmetry

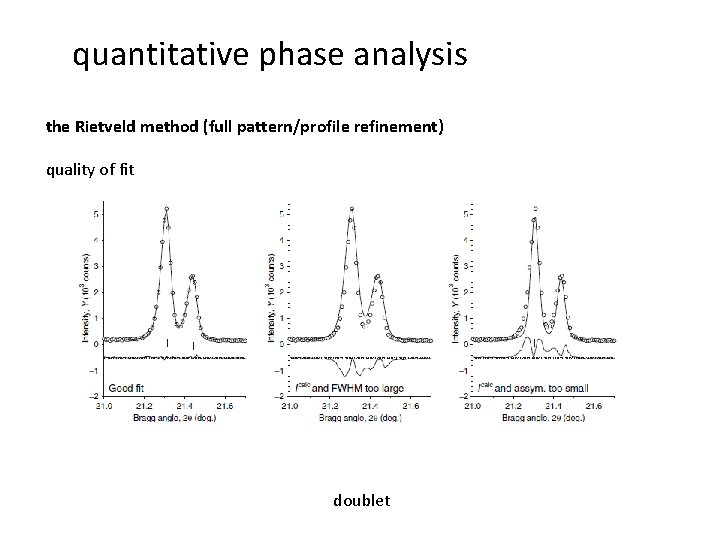
quantitative phase analysis the Rietveld method (full pattern/profile refinement) quality of fit doublet

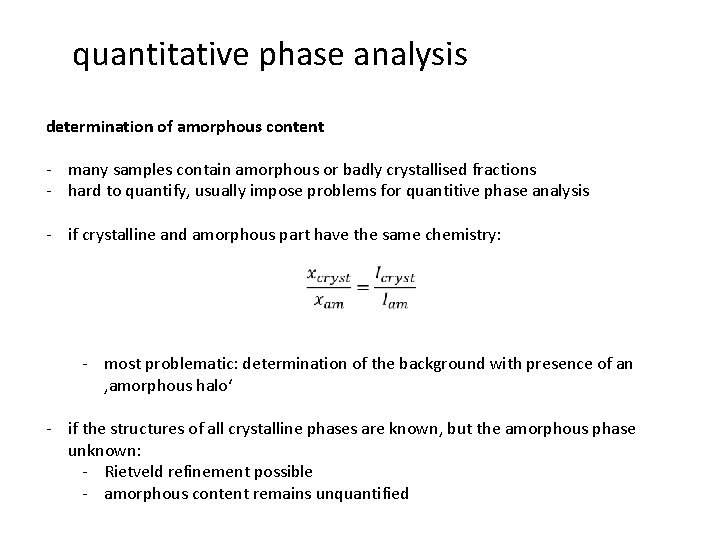
quantitative phase analysis determination of amorphous content - many samples contain amorphous or badly crystallised fractions - hard to quantify, usually impose problems for quantitive phase analysis - if crystalline and amorphous part have the same chemistry: - most problematic: determination of the background with presence of an ‚amorphous halo‘ - if the structures of all crystalline phases are known, but the amorphous phase unknown: - Rietveld refinement possible - amorphous content remains unquantified

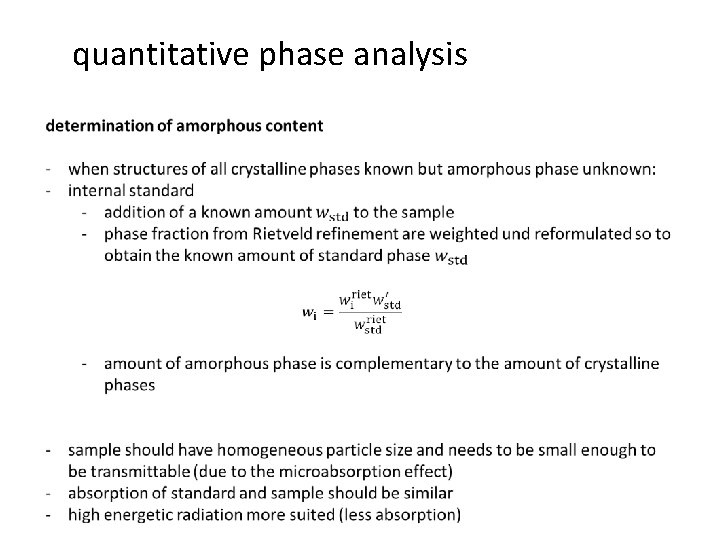
quantitative phase analysis determination of amorphous content - when structures of all crystalline phases known but amorphous phase unknown: - external standard - standard and sample must be measured separately, but with identical conditions - from the scale factor of the two measurements: no amorphous content - amorphous content = non-crystalline fraction - accuracy relies heavily on calculated µm (porosity, packing density, …) - determination of µm is possible through Compton scattering - powder should possess a homogeneous packing density in order to minimise fluctuations in the absorption coefficient between sample and standard

quantitative phase analysis

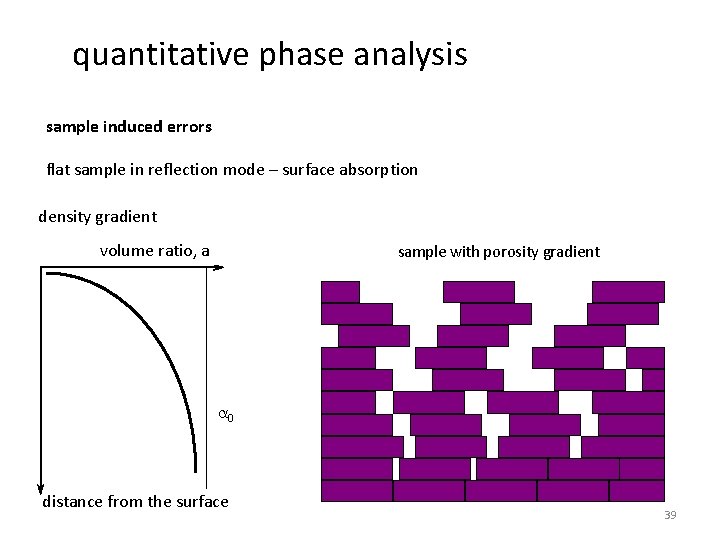
quantitative phase analysis sample induced errors flat sample in reflection mode – surface absorption density gradient volume ratio, a sample with porosity gradient a 0 distance from the surface 39

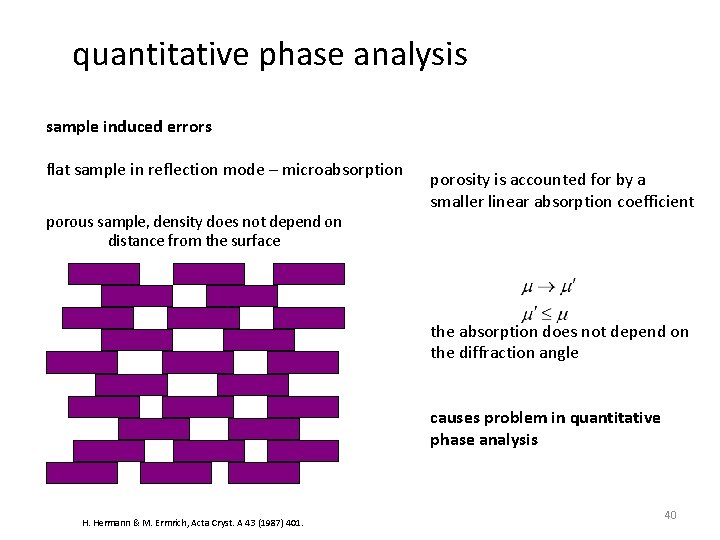
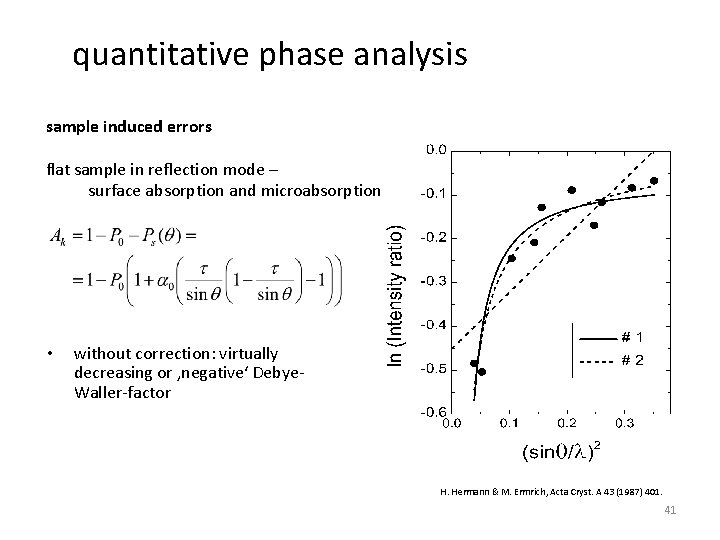
quantitative phase analysis sample induced errors flat sample in reflection mode – microabsorption porous sample, density does not depend on distance from the surface porosity is accounted for by a smaller linear absorption coefficient the absorption does not depend on the diffraction angle causes problem in quantitative phase analysis H. Hermann & M. Ermrich, Acta Cryst. A 43 (1987) 401. 40

quantitative phase analysis sample induced errors flat sample in reflection mode – surface absorption and microabsorption • without correction: virtually decreasing or ‚negative‘ Debye. Waller-factor H. Hermann & M. Ermrich, Acta Cryst. A 43 (1987) 401. 41

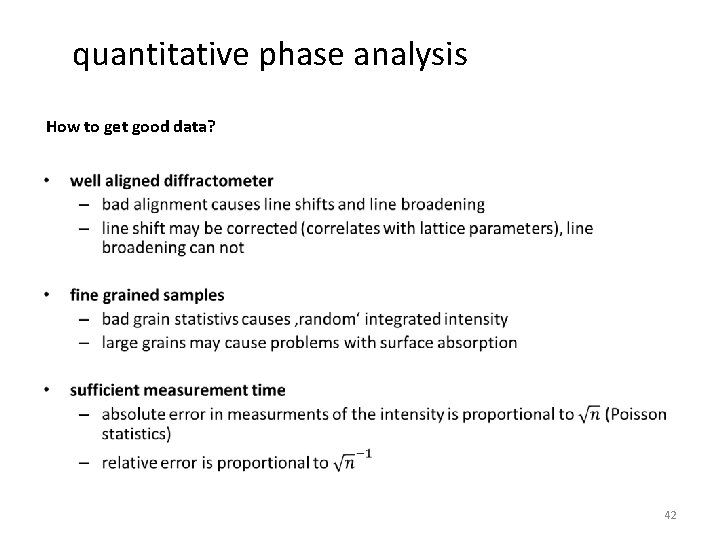
quantitative phase analysis How to get good data? • 42

quantitative phase analysis Do‘s and don‘t‘s? • • • do not refine parameters defining the crystal structure (fractional coordinates, occupancies, thermal motion parameters) – unless there is a good reason to do so refine only parameters being absolutely necessary (the fewer the refinabel parameters, the steeper is the convergence) avoid simultaneous refinement of correlating parameters quality of powder pattern is usually insufficient to estimate anisotropic Debye. Waller factors record powder patterns in an angular range as high as possible to account for different functional dependencies of structural and instrumental parameters on 2 q 43