Quantitative Image Analysis of HER 2 Immunohistochemistry Compared
















- Slides: 28

Quantitative Image Analysis of HER 2 Immunohistochemistry Compared with Manual Pathologist Analysis in Breast Cancer A Pilot Study Keith J. Kaplan, MD Geoffrey L. Turner, MD Ph. D Grace E. Kronauer Northwestern University Feinberg School of Medicine Johns Hopkins University

Background – HER 2 n n n 2 Human epidermal growth factor receptor 2 gene ERBB 2 (commonly referred to as HER 2) is amplified in approximately 18% to 20% of breast cancers. HER 2 overexpression is associated with clinical outcomes in patients with breast cancer. HER 2 status is also predictive for several systemic therapies.

Background n Several studies have now shown that agents that target HER 2 are remarkably effective in both the metastatic and adjuvant settings. Trastuzumab (Herceptin; Genentech, South San Francisco, CA), a humanized monoclonal antibody, improves response rates, time to progression, and even survival when used alone or added to chemotherapy in metastatic breast cancer. n n 3 Active as a single agent. Approve for the treatment of metastatic disease.

Background n HER 2 testing should be routinely performed in patients with a new diagnosis of invasive breast cancer. However, the best method to assess HER 2 status, in regards both to the type of assay used and the optimal method to perform each assay, remains controversial. IHC with reflex of 2+ to FISH n Overexpression by IHC (3+) or amplification by FISH considered positive result n 4

Background 5 n Several assays have been used for HER 2 determination in tissue. US Food and Drug Administration regulations also allow pathology laboratories to develop and implement so called “home brew assays” using US Food and Drug Administration–approved analyte specific reagents. n While some assays have been carefully validated, others, especially “home brew assays, ” have not. Prospective substudies from two of the adjuvant randomized trials of trastuzumab versus nil have demonstrated that approximately 20% of HER 2 assays performed in the field (at the primary treatment site's pathology department) were incorrect when the same specimen was re-evaluated.

Background n n 6 Such a disorganized practice and high rate of inaccuracy, for such an important test that dictates a critically effective yet potentially lifethreatening and expensive treatment, is not acceptable. Trastuzumab therapy is not without its drawbacks. Therapy recommended for 12 months The drug cost of 52 weeks of trastuzumab in the community setting in the United States is approximately $100, 000 based on average sales price (www. accc-cancer. org).

Background n Associated with a small risk of serious cardiac toxicity. n n n 7 Approximately 5% to 15% of patients develop cardiac dysfunction, Approximately 1% to 4% develop significant cardiac events (including symptomatic congestive heart failure) while taking trastuzumab. Taken together, the significant benefits coupled with the high cost and potential cardiotoxicity of trastuzumab demand accurate HER 2 testing.

Archives of Pathology and Laboratory Medicine: Vol. 131, No. 1, pp. 18– 43. 8

9

10

• Image analysis can be an effective tool for achieving consistent interpretation. However, a pathologist must confirm the image analysis result. Image analysis equipment, just as other laboratory equipment, must be calibrated and subjected to regular maintenance and internal quality control evaluation. Image analysis procedures must be validated before implementation. One issue identified during the panel discussion was lack of calibration of the optical microscopes used by pathologists, something which certainly contributes to interpretive variation. If pathologists use several different microscopes to read assays, a system of calibration of these instruments should be implemented to ensure consistent interpretation. 11 Archives of Pathology and Laboratory Medicine: Vol. 131, No. 1, pp. 18– 43.

Inconsistency of HER 2 Test Raises Questions J Natl Cancer Inst 2007; 99(14)1064 -1065. The tests that determine who gets the powerful breast cancer drug trastuzumab (Herceptin) may not be as reliable as previously thought, researchers reported at the annual meeting of the American Society of Clinical Oncology. That means some women who should be getting trastuzumab treatment are not, while other women who will not benefit are unnecessarily exposed to a drug that can cause heart problems. 12

Study Design n n Retrospectively reviewed 81 (81/122) breast cancers newly diagnosed within 2006 and tested for HER 2 by IHC with reflex FISH testing in equivocal (2+) cases (majority) LIS searched for “FISH” and “HER 2” in 2006 Whole slide scanned using Scan. Scope and analyzed using membrane stain algorithm provided Algorithms not “calibrated” or “standardized” to our laboratory. n n n 13 Slides not re-reviewed or IHCs re-scored Non-sequential cases from 10 pathologists Home-brewed assay

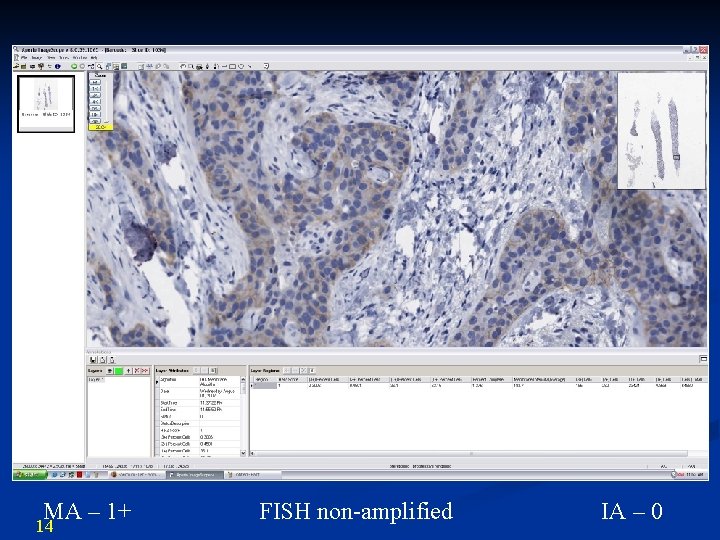
MA – 1+ 14 FISH non-amplified IA – 0

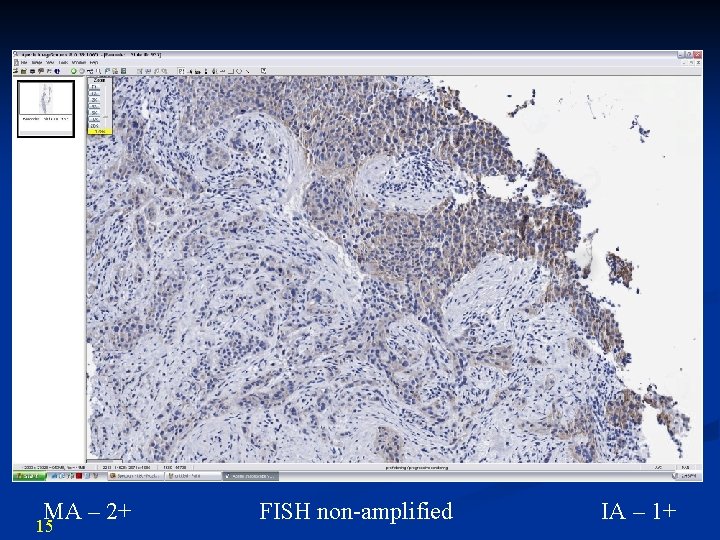
MA – 2+ 15 FISH non-amplified IA – 1+

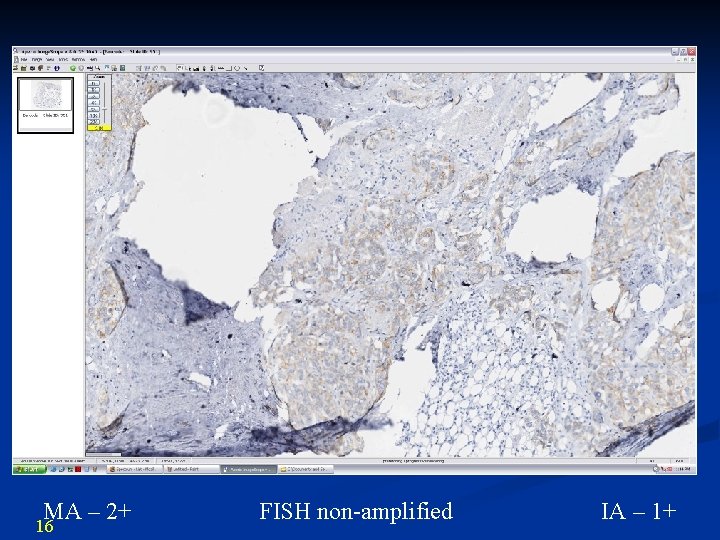
MA – 2+ 16 FISH non-amplified IA – 1+

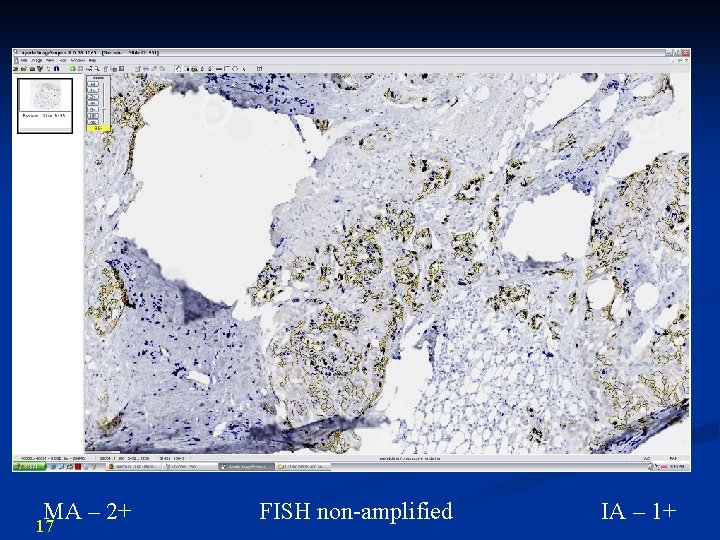
MA – 2+ 17 FISH non-amplified IA – 1+

18

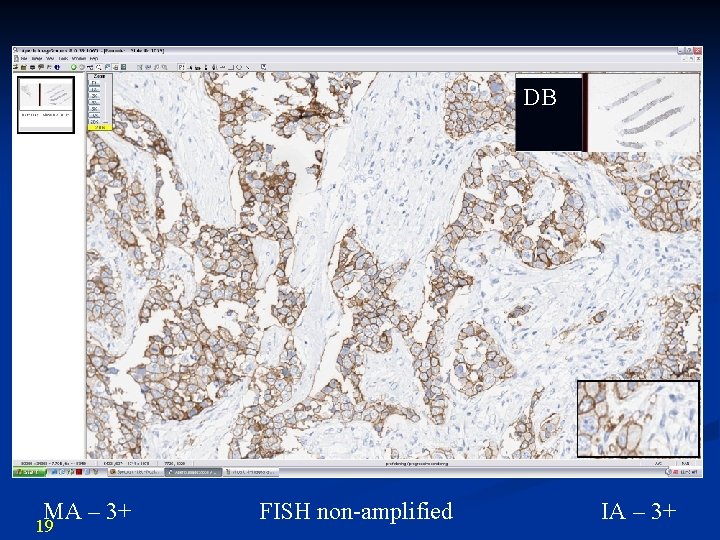
DB MA – 3+ 19 FISH non-amplified IA – 3+

MA – 3+ 20 FISH amplified IA – 3+

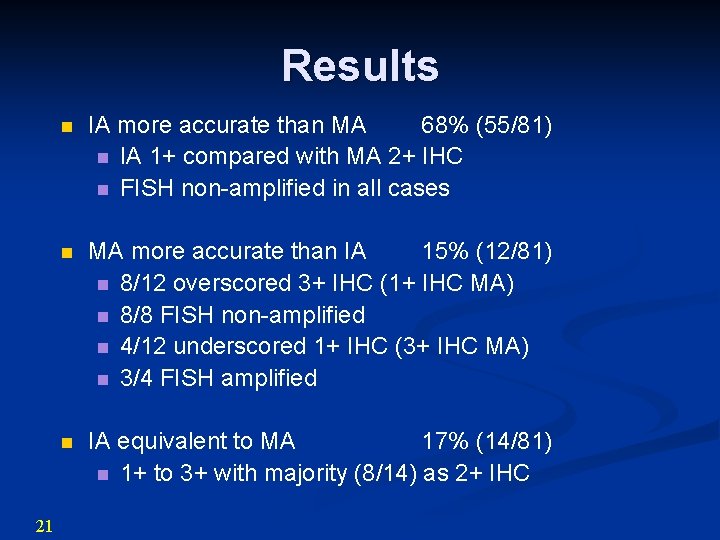
Results 21 n IA more accurate than MA 68% (55/81) n IA 1+ compared with MA 2+ IHC n FISH non-amplified in all cases n MA more accurate than IA 15% (12/81) n 8/12 overscored 3+ IHC (1+ IHC MA) n 8/8 FISH non-amplified n 4/12 underscored 1+ IHC (3+ IHC MA) n 3/4 FISH amplified n IA equivalent to MA 17% (14/81) n 1+ to 3+ with majority (8/14) as 2+ IHC

Results n n Scan time on order of a few minutes Processing time on order of 15 minutes for whole slide 2 slides unable to be scanned Extra resources needed to incorporate into workflow n 22 Retrieval of material, scanning, processing, analyzing and reporting

Caveats n n 23 Cases signed out prior to guidelines in 2007 Home-brewed assay used for IHC Using FISH as gold standard rather than outcome Low threshold for calling indeterminate

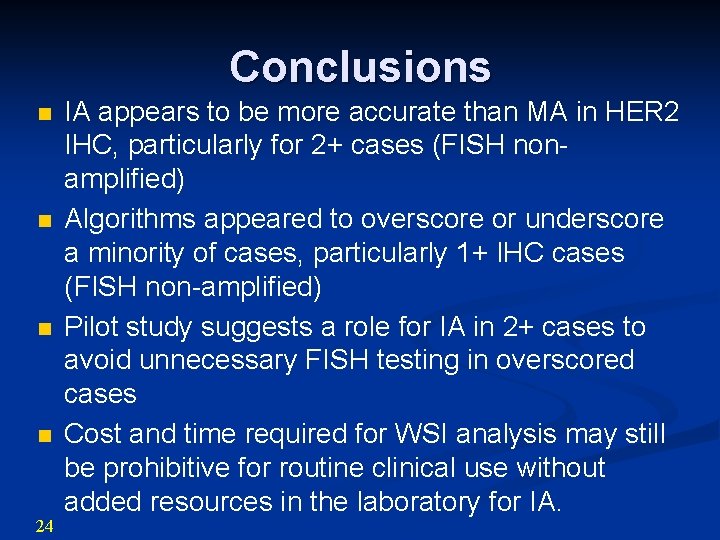
Conclusions n n 24 IA appears to be more accurate than MA in HER 2 IHC, particularly for 2+ cases (FISH nonamplified) Algorithms appeared to overscore or underscore a minority of cases, particularly 1+ IHC cases (FISH non-amplified) Pilot study suggests a role for IA in 2+ cases to avoid unnecessary FISH testing in overscored cases Cost and time required for WSI analysis may still be prohibitive for routine clinical use without added resources in the laboratory for IA.

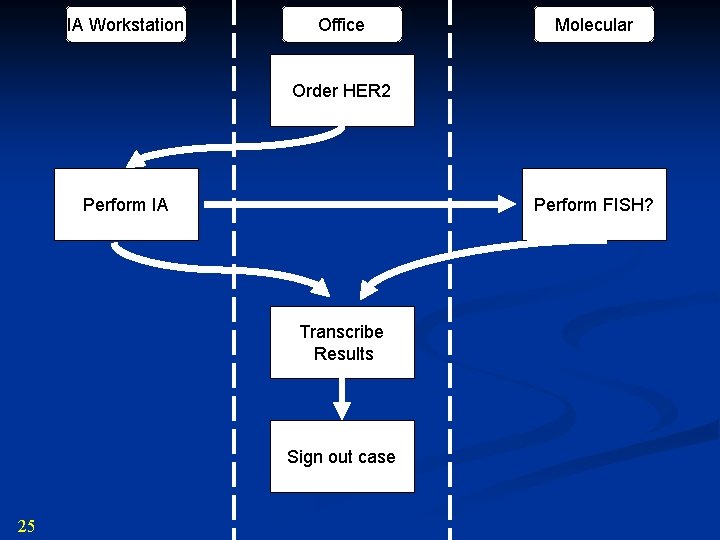
IA Workstation Office Molecular Order HER 2 Perform IA Perform FISH? Transcribe Results Sign out case 25

Conclusions n CPT 88360 – Morphometric analysis, tumor immunohistochemistry (eg, Her 2/neu, estrogen/progesterone receptor), quantitative, semiquantitative, each antibody; manual n n CPT 88361 – Morphometric analysis, tumor immunohistochemistry (eg, Her 2/neu, estrogen/progesterone receptor), quantitative, semiquantitative, each antibody; using computer assisted technology n n 88361 PC+TC=$229. 16 Δ(TC+PC)=$81. 21 HER 2 FISH § 26 PC+TC=$147. 95 88368 PC+TC=$710. 00

Acknowledgments n n n 27 Aperio Technologies, Inc. Laura Nottoli, Craig Fenstermaker, Holger Lange Grace Kronauer Geoffrey Turner MD Ph. D Tracy Roberts

Thank You 28 www. tissuepathology. com