Q Which weighs more A kilogram of feathers



- Slides: 9

Q) Which weighs more: A kilogram of feathers or a kilogram of iron? DENSITY

What is Density? The same volume of different substances WILL have different masses. Wood Water Iron 1 cm 3 0. 50 g 1. 00 g 8. 00 g IRON Q) Which has the greatest mass and therefore the most dense? Density is the Mass per unit Volume

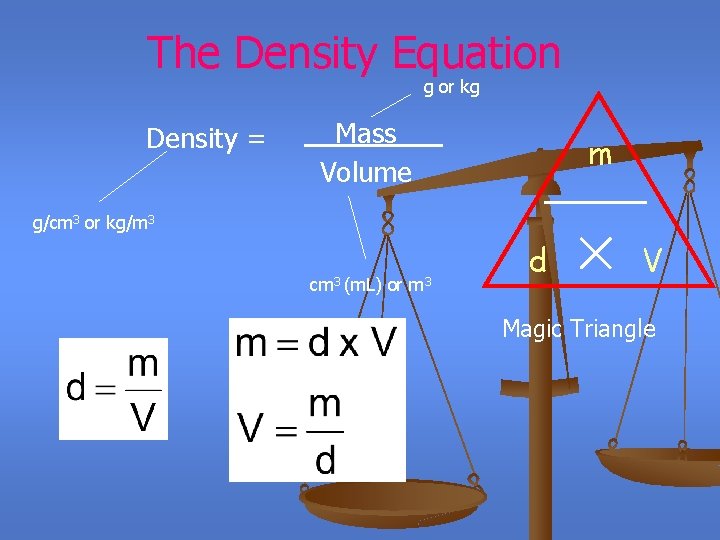
The Density Equation g or kg Density = __Mass___ Volume m g/cm 3 or kg/m 3 cm 3 (m. L) or m 3 d V Magic Triangle

Density Calculations Example: Liquid water has a density of 1000 kg/m 3, while ice has density of 920 kg/m 3. Calculate the volume occupied by 0. 25 kg of each. V = m = ___0. 25 kg____ = 0. 000250 m 3 d 1000 kg/m 3 V = m = ___0. 25 kg____ = 0. 000272 m 3 d 920 kg/m 3

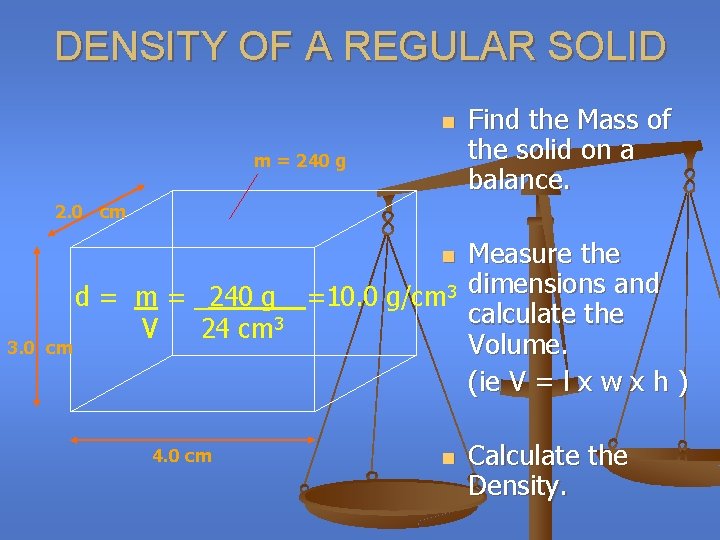
DENSITY OF A REGULAR SOLID n m = 240 g Find the Mass of the solid on a balance. 2. 0 cm Measure the d = m = _240 g__=10. 0 g/cm 3 dimensions and calculate the 3 V 24 cm Volume. cm (ie V = l x w x h ) n 3. 0 4. 0 cm n Calculate the Density.

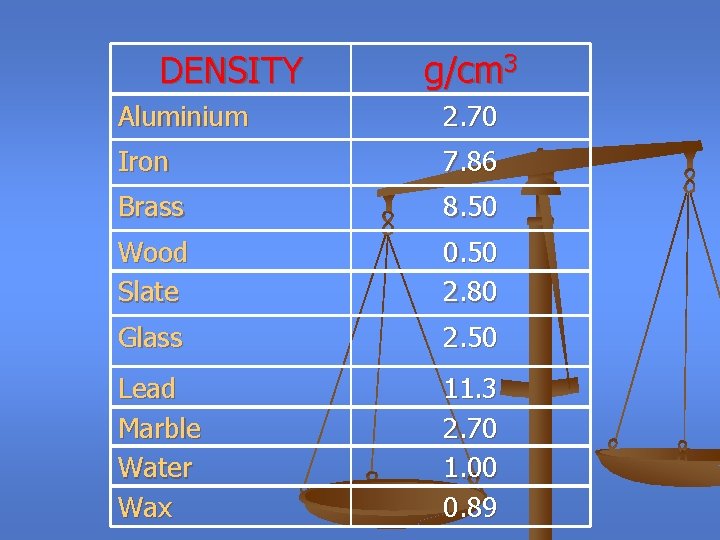
DENSITY g/cm 3 Aluminium 2. 70 Iron 7. 86 Brass 8. 50 Wood Slate 0. 50 2. 80 Glass 2. 50 Lead Marble Water Wax 11. 3 2. 70 1. 00 0. 89

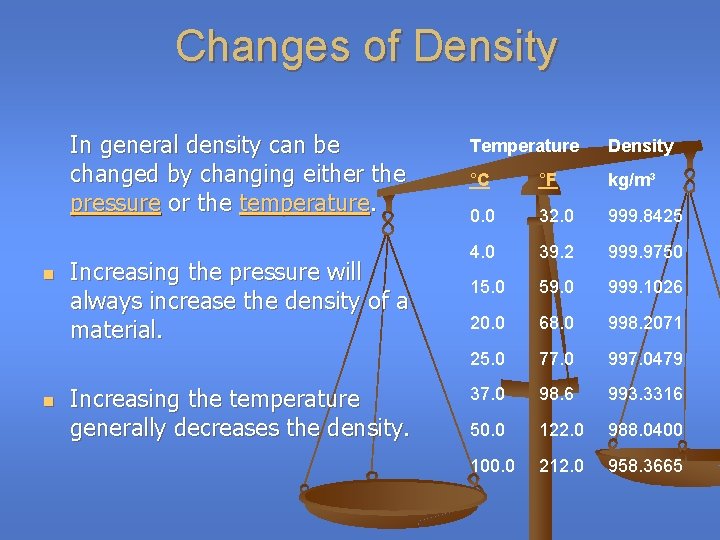
Changes of Density In general density can be changed by changing either the pressure or the temperature. n n Increasing the pressure will always increase the density of a material. Increasing the temperature generally decreases the density. Temperature Density °C °F kg/m³ 0. 0 32. 0 999. 8425 4. 0 39. 2 999. 9750 15. 0 59. 0 999. 1026 20. 0 68. 0 998. 2071 25. 0 77. 0 997. 0479 37. 0 98. 6 993. 3316 50. 0 122. 0 988. 0400 100. 0 212. 0 958. 3665

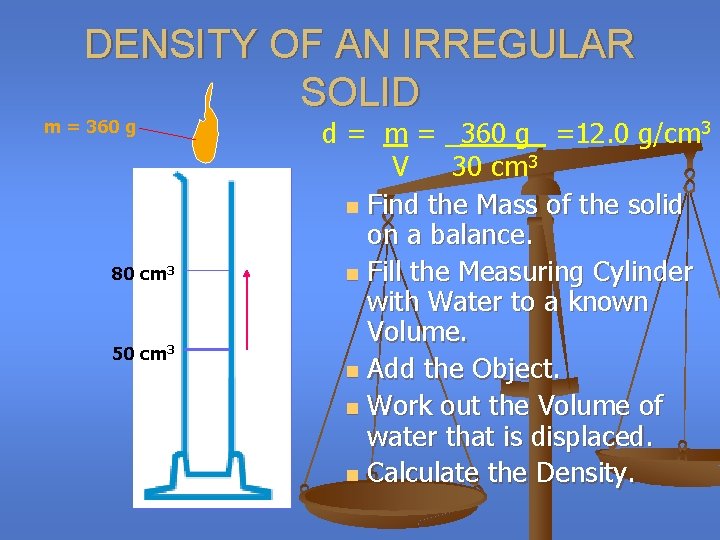
DENSITY OF AN IRREGULAR SOLID m = 360 g 80 cm 3 50 cm 3 d = m = _360 g_ =12. 0 g/cm 3 V 30 cm 3 n Find the Mass of the solid on a balance. n Fill the Measuring Cylinder with Water to a known Volume. n Add the Object. n Work out the Volume of water that is displaced. n Calculate the Density.

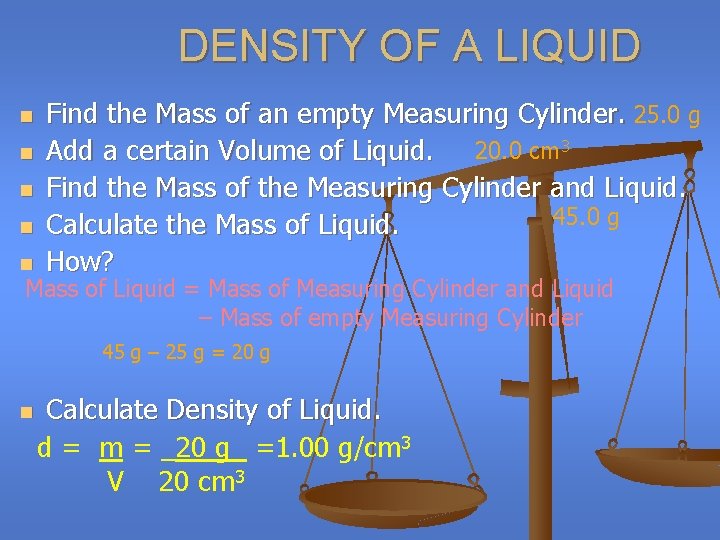
DENSITY OF A LIQUID n n n Find the Mass of an empty Measuring Cylinder. 25. 0 g Add a certain Volume of Liquid. 20. 0 cm 3 Find the Mass of the Measuring Cylinder and Liquid. 45. 0 g Calculate the Mass of Liquid. How? Mass of Liquid = Mass of Measuring Cylinder and Liquid – Mass of empty Measuring Cylinder 45 g – 25 g = 20 g n Calculate Density of Liquid. d = m = _20 g_ =1. 00 g/cm 3 V 20 cm 3
 Kilogram of feathers
Kilogram of feathers A kilogram of feathers
A kilogram of feathers More more more i want more more more more we praise you
More more more i want more more more more we praise you More more more i want more more more more we praise you
More more more i want more more more more we praise you What weighs 1 kilogram
What weighs 1 kilogram What is the weight of a paperclip
What is the weight of a paperclip Density of water in kg/m3
Density of water in kg/m3 A toy rocket that weighs 10 n
A toy rocket that weighs 10 n Bir tabak pirincin kütlesi
Bir tabak pirincin kütlesi The diagram below represents a 155 newton box on a ramp
The diagram below represents a 155 newton box on a ramp