Density Q Which weighs more A kilogram of








- Slides: 17

Density

Q) Which weighs more: A kilogram of feathers or a kilogram of iron? DENSITY Objective: To describe how to determine density using direct measurements of mass and volume

What is Density? If you take the same volume of different substances, then they will weigh different amounts. 1 Wood Water cm 3 0. 50 g 1 1. 00 g Iron 1 cm 3 = 1 ml 8. 00 g Q) Which has the greatest mass and therefore the most dense? Density is the Mass per unit Volume

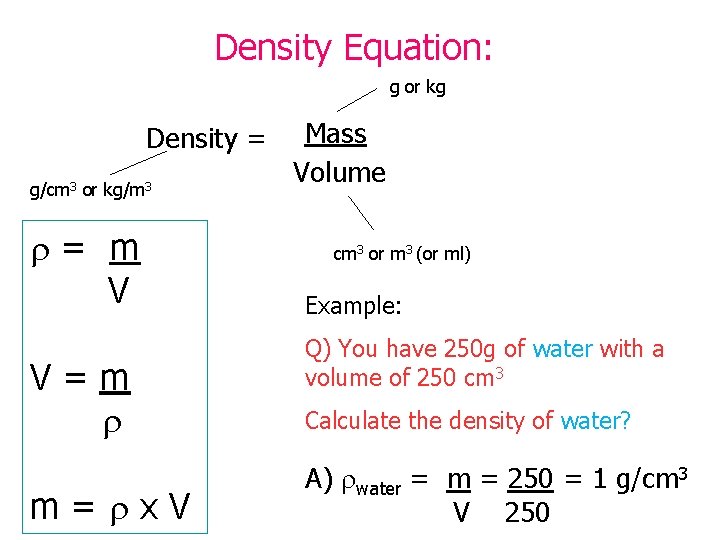
Density Equation: g or kg Density = g/cm 3 or kg/m 3 = m V V=m m= x. V Mass Volume cm 3 or m 3 (or ml) Example: Q) You have 250 g of water with a volume of 250 cm 3 Calculate the density of water? A) water = m = 250 = 1 g/cm 3 V 250

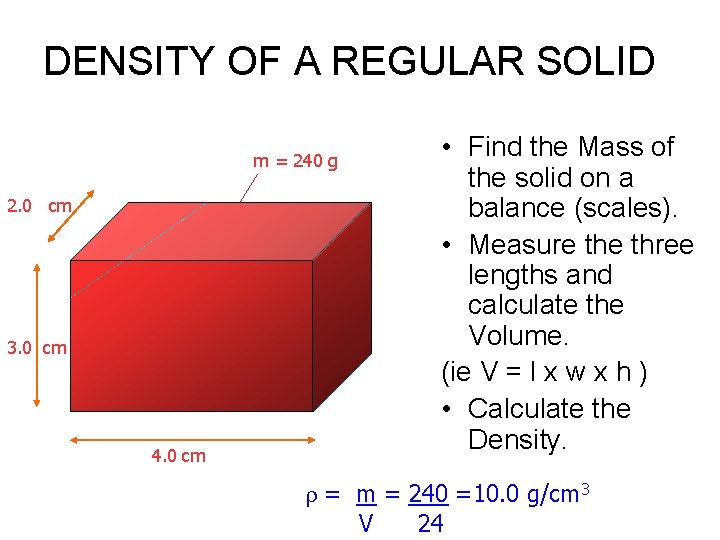
DENSITY OF A REGULAR SOLID m = 240 g 2. 0 cm 3. 0 cm 4. 0 cm • Find the Mass of the solid on a balance (scales). • Measure three lengths and calculate the Volume. (ie V = l x w x h ) • Calculate the Density. = m = 240 =10. 0 g/cm 3 V 24

DENSITY OF AN IRREGULAR SOLID • Find the Mass of the solid on a balance. • Find the Volume. • Calculate the Density.

Archimedes and the Crown Archimedes was a great scientist who lived more than 2000 years ago in a Greek city called Syracuse. He was very clever at inventing and figuring out how things work, so King Hiero needed his help many times.

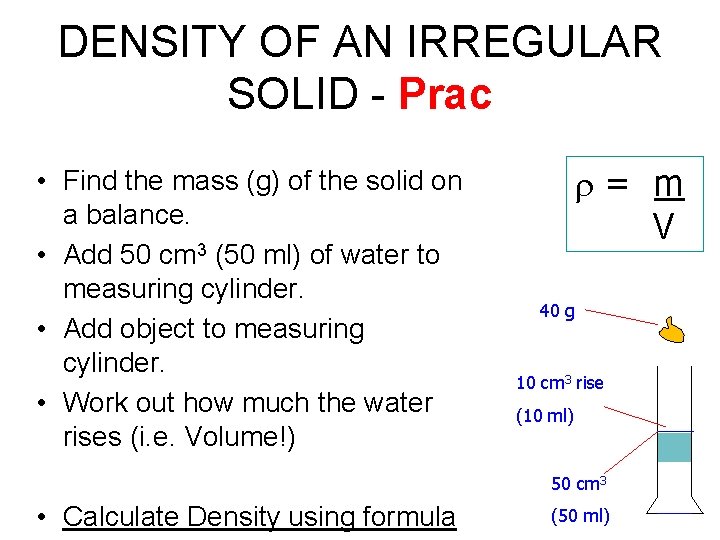
DENSITY OF AN IRREGULAR SOLID - Prac • Find the mass (g) of the solid on a balance. • Add 50 cm 3 (50 ml) of water to measuring cylinder. • Add object to measuring cylinder. • Work out how much the water rises (i. e. Volume!) = m V 40 g 10 cm 3 rise (10 ml) 50 cm 3 • Calculate Density using formula (50 ml)

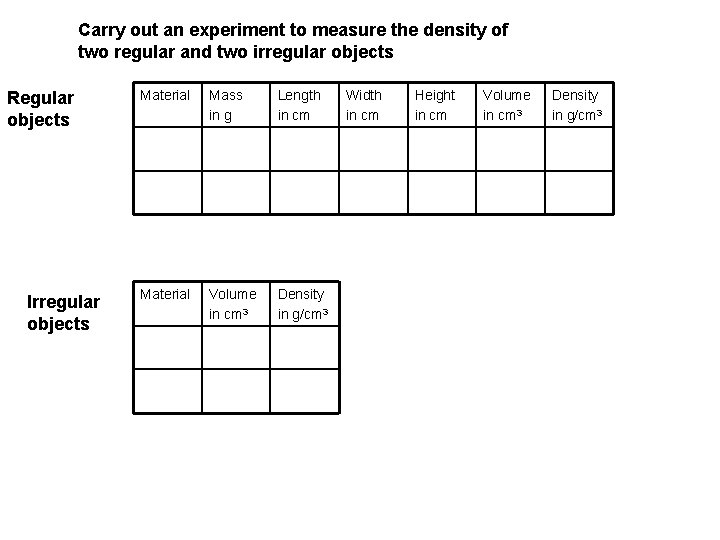
Carry out an experiment to measure the density of two regular and two irregular objects Regular objects Irregular objects Material Mass in g Length in cm Material Volume in cm 3 Density in g/cm 3 Width in cm Height in cm Volume in cm 3 Density in g/cm 3

Stop! Quiz Time!

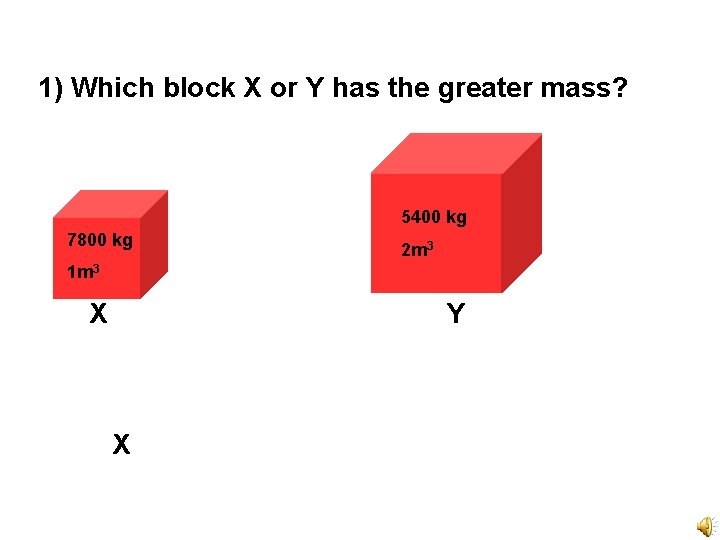
1) Which block X or Y has the greater mass? 5400 kg 7800 kg 2 m 3 1 m 3 X Y X

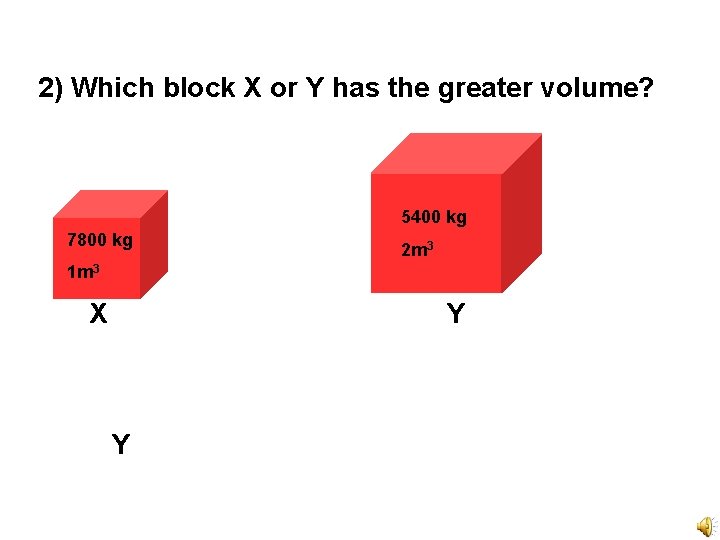
2) Which block X or Y has the greater volume? 5400 kg 7800 kg 2 m 3 1 m 3 X Y Y

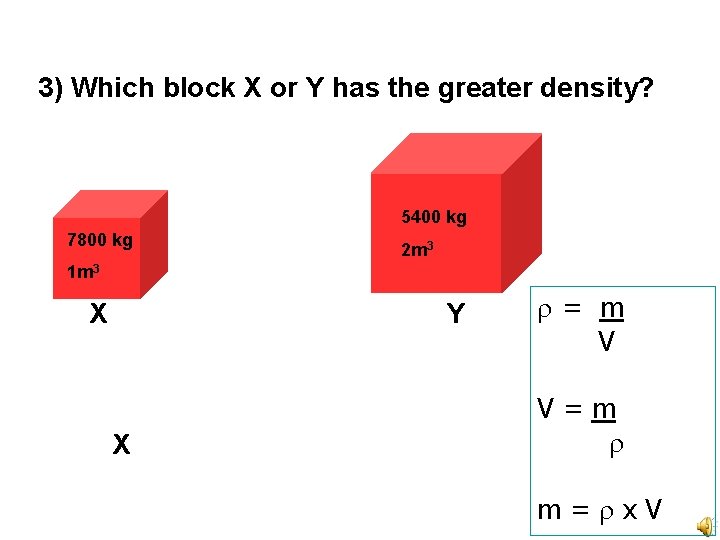
3) Which block X or Y has the greater density? 5400 kg 7800 kg 2 m 3 1 m 3 X Y X = m V V=m m= x. V

Density of Petrol = 800 kg/m 3 Density of water = 1000 kg/m 3 4) Which has more mass 1 m 3 of petrol of 1 m 3 of water? 1 m 3 of water

Density of Petrol = 800 kg/m 3 Density of water = 1000 kg/m 3 5) Which has more volume (takes up more space) 1 kg of petrol or 1 kg of water? 1 kg of petrol

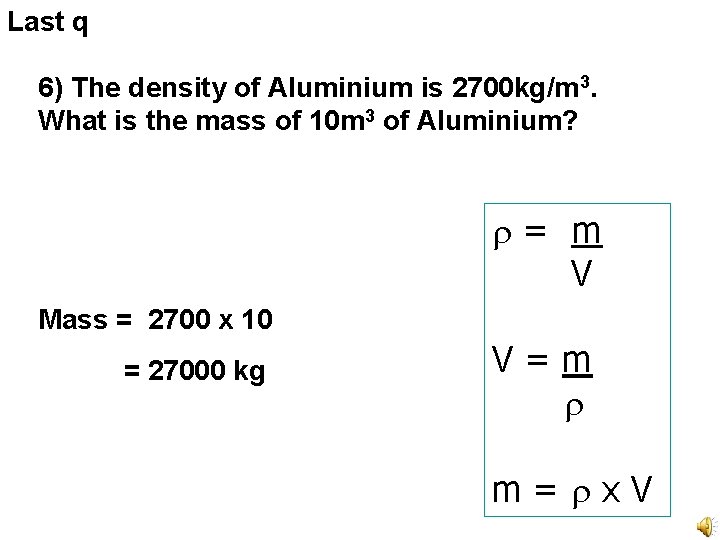
Last q 6) The density of Aluminium is 2700 kg/m 3. What is the mass of 10 m 3 of Aluminium? = m V Mass = 2700 x 10 = 27000 kg V=m m= x. V

Homework DENSITY Objective: To describe how to determine density using direct measurements of mass and volume. Homework: Read pages 10 -11 in the textbook and answer the questions Optional Activity: https: //phet. colorado. edu/sims/density-andbuoyancy/density_en. html