Psychrometrics Introduction to Food Engineering Psychrometrics n Thermodynamic


















- Slides: 30

Psychrometrics Introduction to Food Engineering

Psychrometrics n Thermodynamic properties of gas-vapor mixtures (air-water(

Properties of Dry Air n Composition n N 2 78 %, O 2 20. 9 % Standard dry air MW = 28. 9645 Gas constant for dry air Ra = 287. 055 m 3. Pa/kg. K


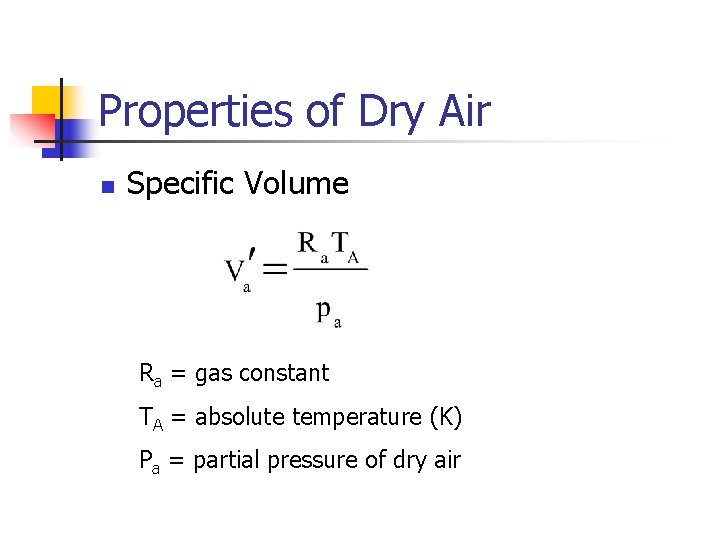
Properties of Dry Air n Specific Volume Ra = gas constant TA = absolute temperature (K) Pa = partial pressure of dry air

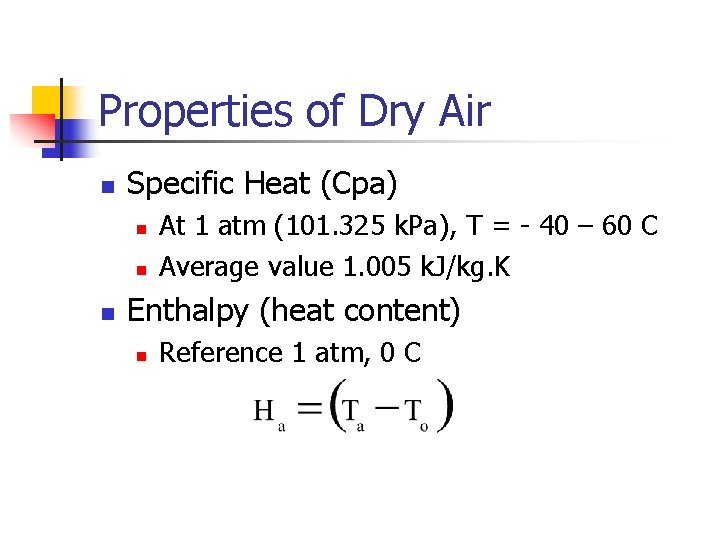
Properties of Dry Air n Specific Heat (Cpa) n n n At 1 atm (101. 325 k. Pa), T = - 40 – 60 C Average value 1. 005 k. J/kg. K Enthalpy (heat content) n Reference 1 atm, 0 C

Properties of Dry Air n Dry Bulb Temperature n Indicated by sensor

Properties of Water Vapor n n n Moist air = dry air + water vapor Vapor in the air is superheated steam at low pressure & temperature Moist air is clear or foggy MW of water = 18. 01534 Gas constant for water vapor Rw = 461. 52 m 3 Pa/kg. K

Properties of Water Vapor n Specific Volume of Water Vapor n Below 66 C vapor follows ideal gas law

Properties of Water Vapor n Specific Heat of Water Vapor n n Within – 71 to 184 C Cpw = 1. 88 k. J/kg. K Enthalpy of water vapor Ta = dry bulb temp

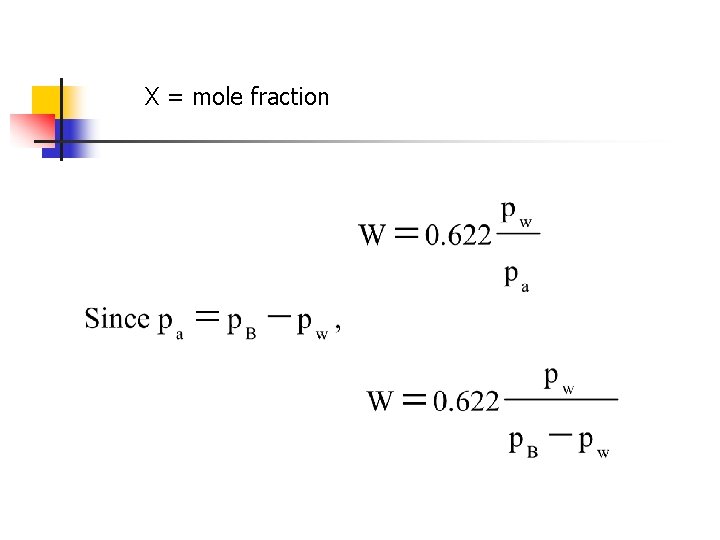
Properties of Air-Vapor Mixtures n Gibbs-Dalton Law n Up to 3 atm air-water mixtures follow perfect gas laws PB = barometric (total pressure) of moist air (k. Pa)

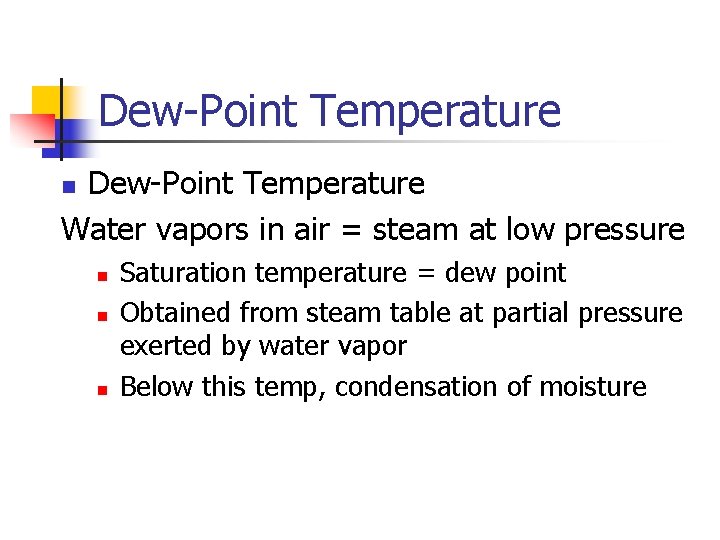
Dew-Point Temperature Water vapors in air = steam at low pressure n n Saturation temperature = dew point Obtained from steam table at partial pressure exerted by water vapor Below this temp, condensation of moisture

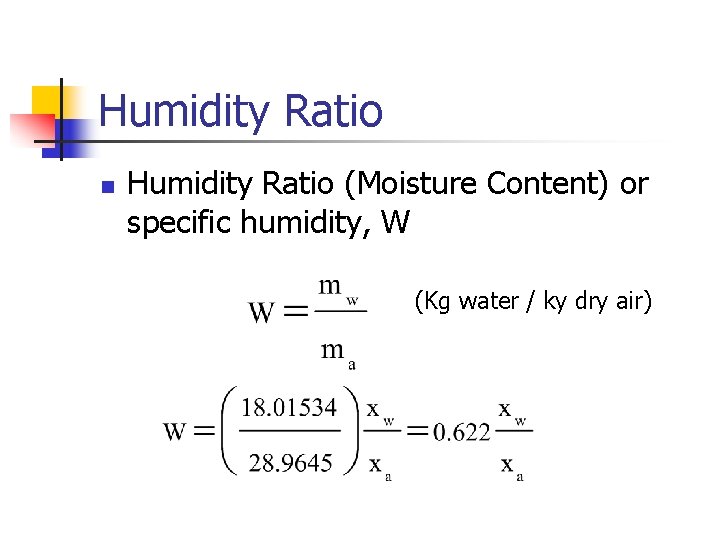
Humidity Ratio n Humidity Ratio (Moisture Content) or specific humidity, W (Kg water / ky dry air)

X = mole fraction

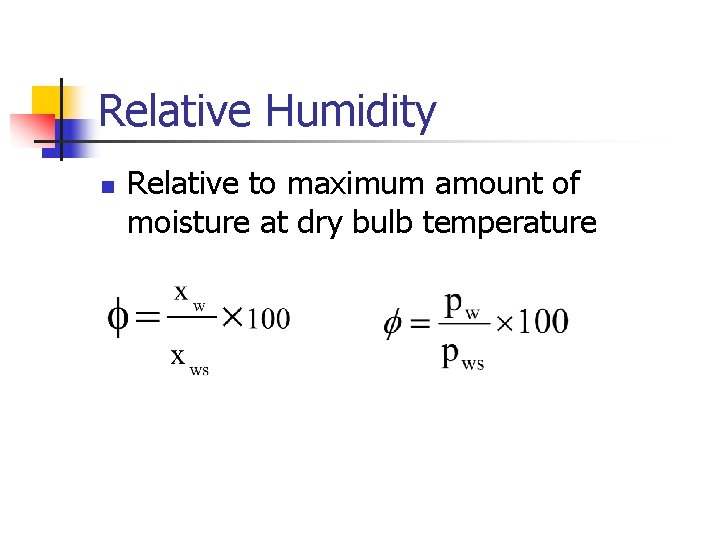
Relative Humidity n Relative to maximum amount of moisture at dry bulb temperature

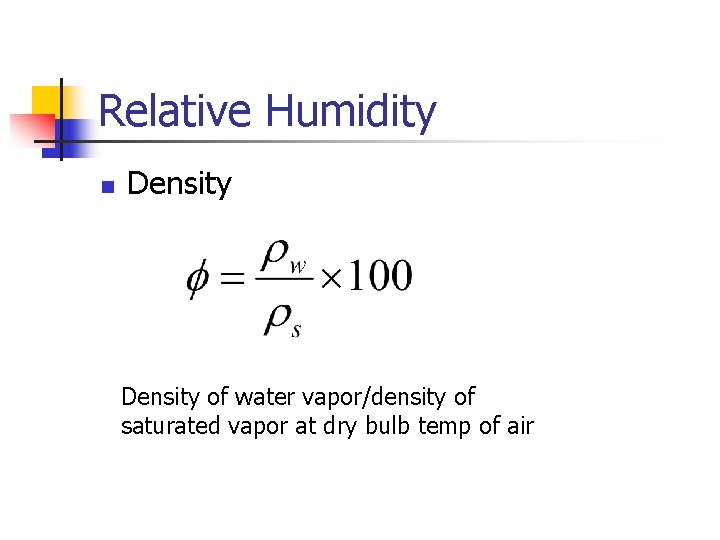
Relative Humidity n Density of water vapor/density of saturated vapor at dry bulb temp of air

Humid Heat n n Of air-water vapor mixture Heat required to raise temp of 1 kg dry air + water vapor by 1 K (k. J/kg dry air. K) W = humidity ratio

Specific Volume n Volume of 1 kg dry air + water vapor

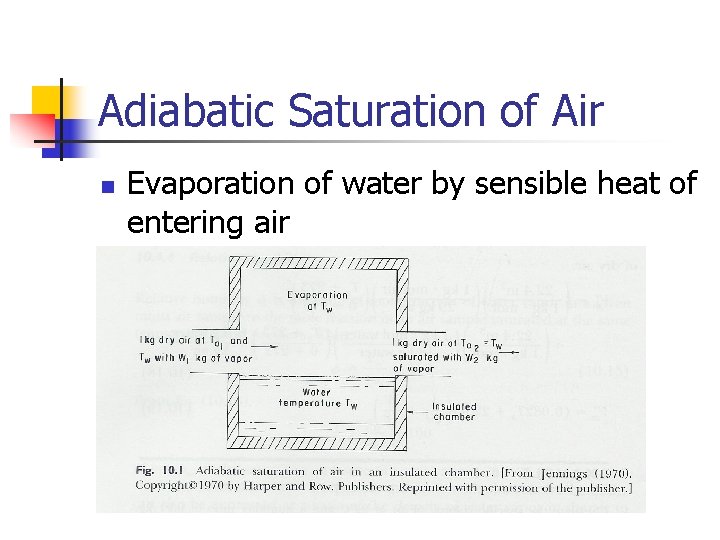
Adiabatic Saturation of Air n Evaporation of water by sensible heat of entering air


Wet-Bulb Temperature n Psychrometric wet bulb temperature n n Movement of air Thermodynamic wet bulb temperature

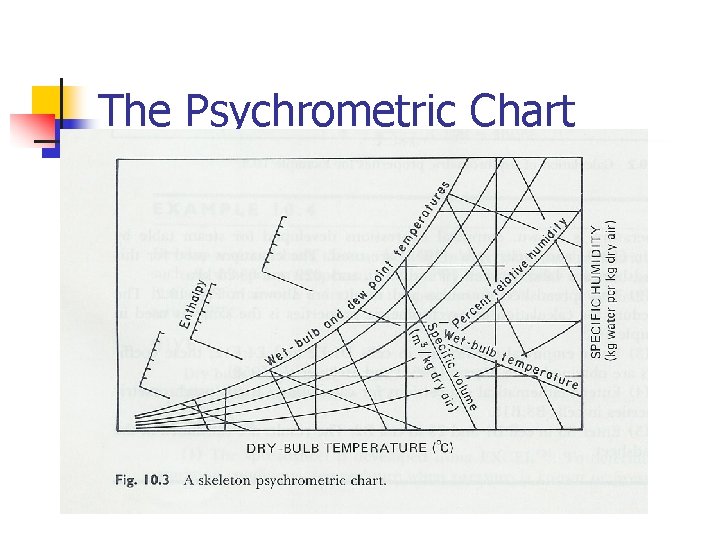
The Psychrometric Chart

Example n An air-vapor mixture is at 60 C dry bulb temp and 35 C wet bulb. Determine relative humidity, humidity ratio, specific volume, enthalpy and dew-point temp. n n RH = 20 %, W = 0. 026 kg/kg Enthalpy = 129 k. J/kg dry air Specific volume = 0. 98 m 3/kg dry air Dew-point temp = 29 C

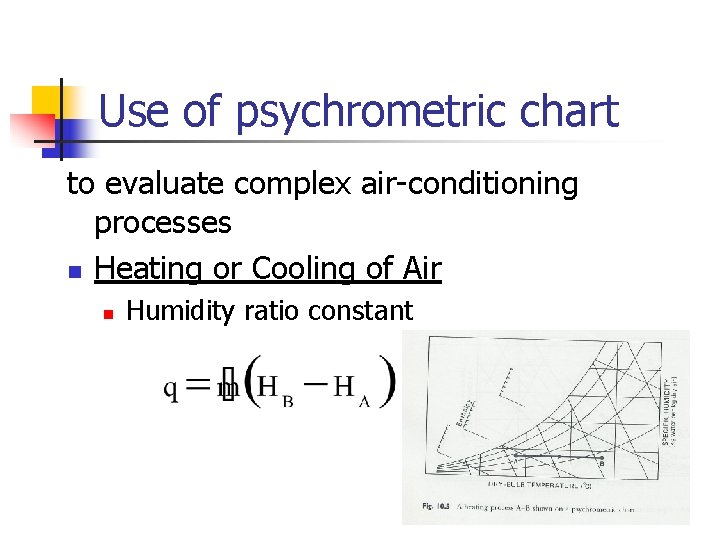
Use of psychrometric chart to evaluate complex air-conditioning processes n Heating or Cooling of Air n Humidity ratio constant

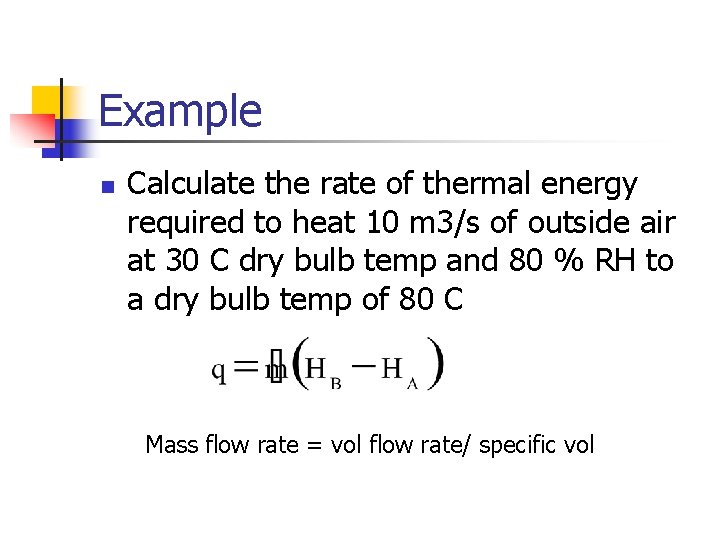
Example n Calculate the rate of thermal energy required to heat 10 m 3/s of outside air at 30 C dry bulb temp and 80 % RH to a dry bulb temp of 80 C Mass flow rate = vol flow rate/ specific vol

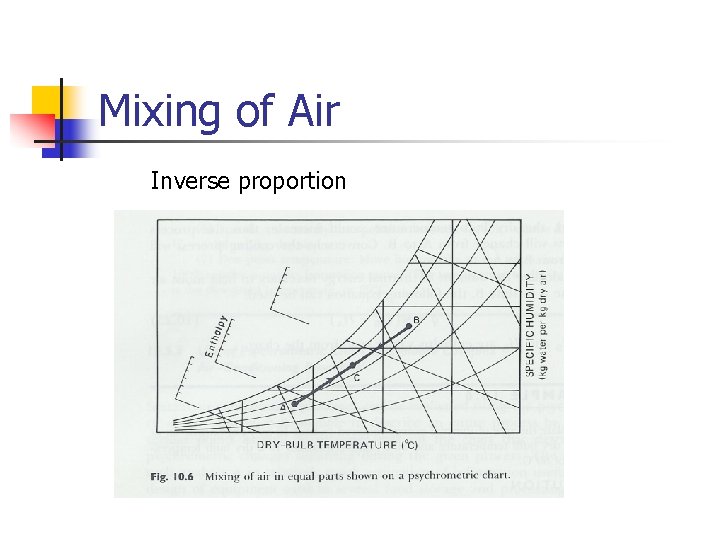
Mixing of Air Inverse proportion

Drying n Adiabatic saturation process n n n Heat of evaporation is supplied only by drying air Dry bulb temp decreases, enthalpy constant ie. constant wet bulb Humidity ratio increases (gain moisture)


Example n Heated air at 50 C, 10 % RH is used to dry rice. Air exits under saturated condition. Determine amount of water removed per kg of dry air. n n W 1 = 0. 0078 kg/kg Follow constant enthalpy line W 2 = 0. 019 kg/kg Moisture removed = 0. 0112 kg/kg

 Psychrometry in food processing
Psychrometry in food processing Indirect contact freezing example
Indirect contact freezing example Unit 2 food food food
Unit 2 food food food Food chain sequence
Food chain sequence Thermodynamic vs kinetic control
Thermodynamic vs kinetic control Thermodynamic probability ppt
Thermodynamic probability ppt Dérivé ln
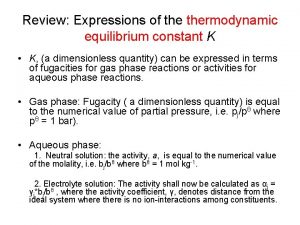
Dérivé ln Equilibrium constant in thermodynamics
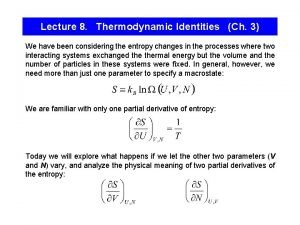
Equilibrium constant in thermodynamics Thermodynamic identity
Thermodynamic identity Thermodynamic behaviour of ideal bose gas
Thermodynamic behaviour of ideal bose gas Cp-cv=r/m
Cp-cv=r/m Gibbs free energy equation
Gibbs free energy equation Thermodynamic property relations
Thermodynamic property relations Thermodynamics 1 formula sheet
Thermodynamics 1 formula sheet Explain helmholtz electrical double layer
Explain helmholtz electrical double layer Thermodynamic
Thermodynamic Volume expansivity and isothermal compressibility
Volume expansivity and isothermal compressibility Entropy in thermodynamics
Entropy in thermodynamics In thermodynamics
In thermodynamics Thermodynamic behaviour of ideal bose gas
Thermodynamic behaviour of ideal bose gas Thermodynamic property relations
Thermodynamic property relations Thermodynamic process
Thermodynamic process Zeroth law of thermodynamics definition
Zeroth law of thermodynamics definition Second law of thermodynamic
Second law of thermodynamic Thermodynamic temperature
Thermodynamic temperature Maxwell relation
Maxwell relation Second law of thermodynamic
Second law of thermodynamic Change of entropy formula
Change of entropy formula Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s Organic chemistry
Organic chemistry Thermodynamic control
Thermodynamic control