Process Urvish Soni Unit Process A unit process













- Slides: 28

Process Urvish Soni

Unit Process • A ''unit process'' is one or more grouped operations in a manufacturing system that can be defined and separated from others.

Unit Process • Chemical processes usually have three interrelated elementary processes 1. Transfer of reactants to the reaction zone 2. Chemical reactions involving various unit processes. 3. Separation of the products from the reaction zone using various unit operations

Importance • Unit Process can be seen as multiple operation combined in a plant • Overall Finance and production planning can be done • Realistic targets to be set • Manpower requirement can be calculated

Cracking • In this process by cracking Large atomic structure into small atomic structure by hit and thermal energy.

Cracking 1. 2. 3. 4. Steam cracking Thermal cracking Hydro cracking Fluid Catalytic cracking or Cat Cracking https: //www. youtube. com/watch? v=7 KO 3 X_8 Qhs. A&t=110 s

Reforming • Reforming is chemical process to convert hydro carbon heaving law octane rating into high octane rating is called reforming. • Naphtha from by reforming we can get high octane gasoline. https: //www. youtube. com/watch? v=Vof. KBcd. Ztjo Video Start with 6: 51

Octane rating

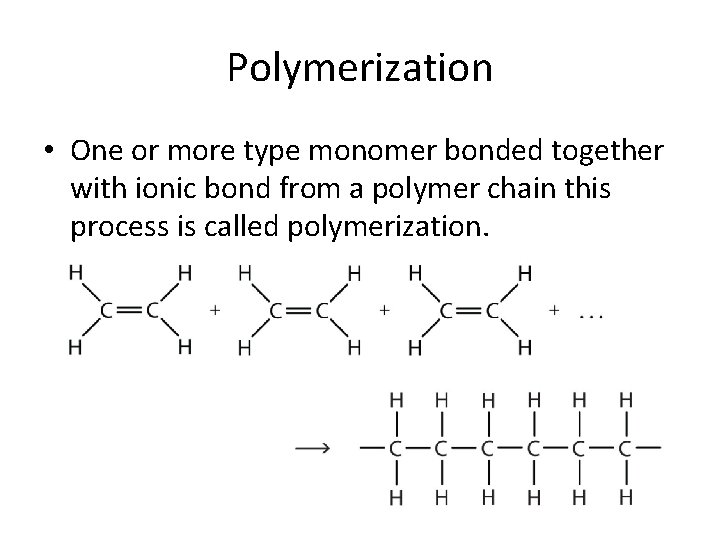
Polymerization • One or more type monomer bonded together with ionic bond from a polymer chain this process is called polymerization.

Polymers • Polythin • P. V. C. • Polystearin https: //www. youtube. com/watch? v=0 y. OJaj. PJpt. A

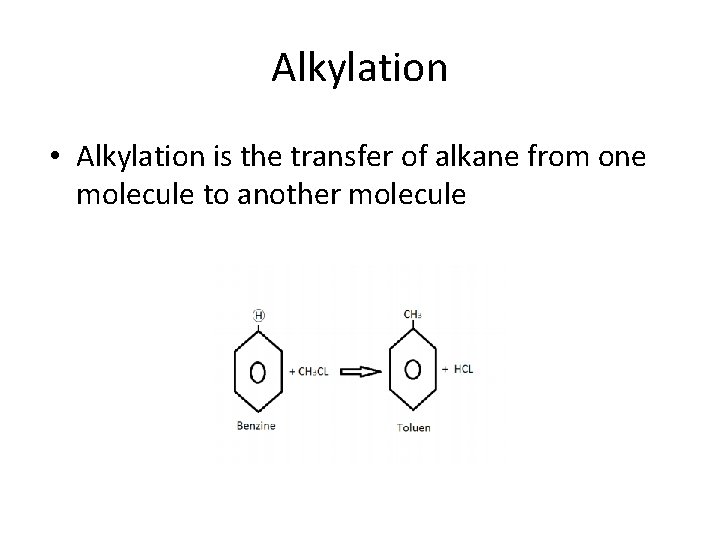
Alkylation • Alkylation is the transfer of alkane from one molecule to another molecule

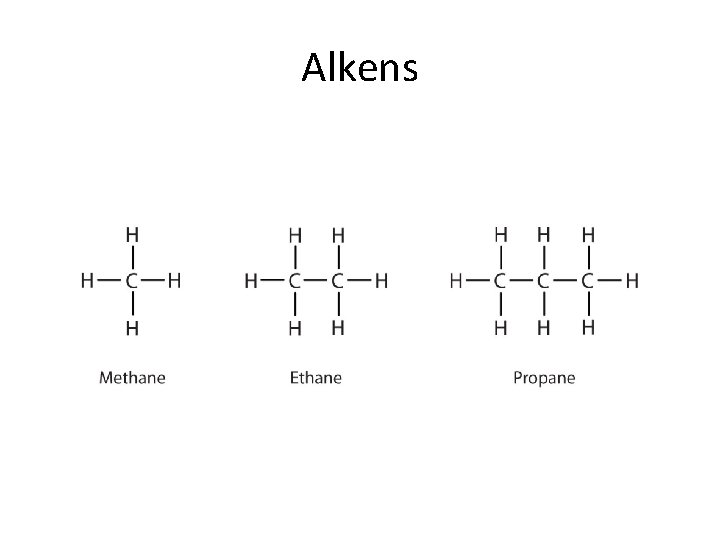
Alkens

Application • In which alkalization of D. N. A is use chemotherapy to damage cancer cell

Hydrogenation • Hydrogenation – meaning, to treat with hydrogen – • It is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. https: //www. youtube. com/watch? v=2 i. Kczq. O 1 Ti. E


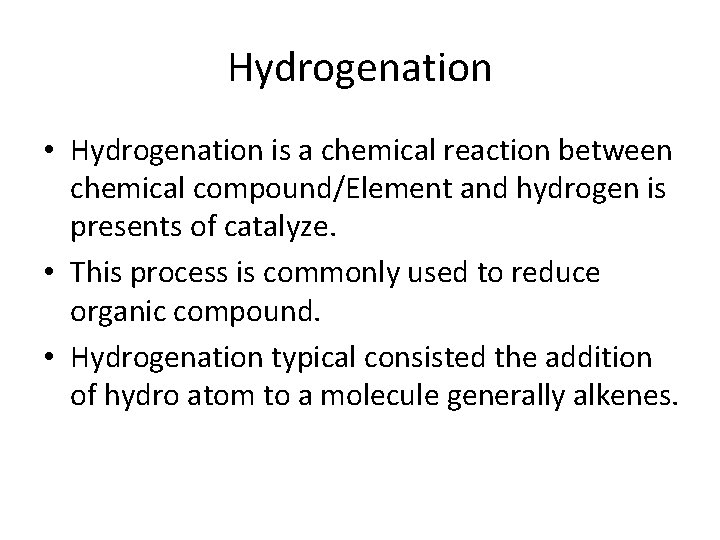
Hydrogenation • Hydrogenation is a chemical reaction between chemical compound/Element and hydrogen is presents of catalyze. • This process is commonly used to reduce organic compound. • Hydrogenation typical consisted the addition of hydro atom to a molecule generally alkenes.

Applications • Food industry – Hydrogenation converts liquid vegetable oils into solid or semi-solid fats • Petrochemical industry – In petrochemical processes, hydrogenation is used to convert alkenes and aromatics into saturated alkanes (paraffins) and cycloalkanes (naphthenes), which are less toxic and less reactive.

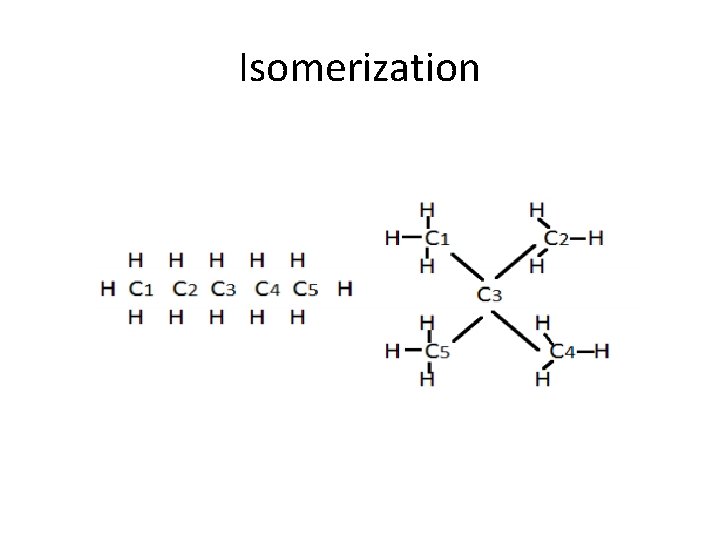
Isomerization • Isomer is the process by which one molecule is transfer to another molecule which has exactly same atom but atoms are rearranged • -A-B-C -> C-B-A -> B-C-A • This is related molecule is known as isomer.

Isomerization

Isomerization • Isomerzation is hydro carbon cracking in organic chemistry where fuels such as pentane isomer are hilted in presents of platinum catalysts this process is isomerization.

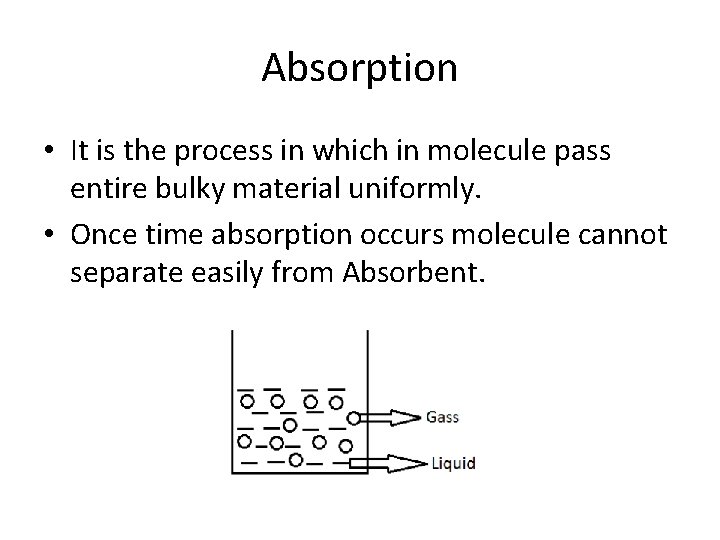
Absorption • It is the process in which in molecule pass entire bulky material uniformly. • Once time absorption occurs molecule cannot separate easily from Absorbent.

Absorption • Examples : – Absorption of perfume molecule by skin – CO 2 Mixture in water as Soda • Applications : – Cooling application – Ice production – Cold storage https: //www. youtube. com/watch? v=dj. Iz. Xvw. Iz 5 U

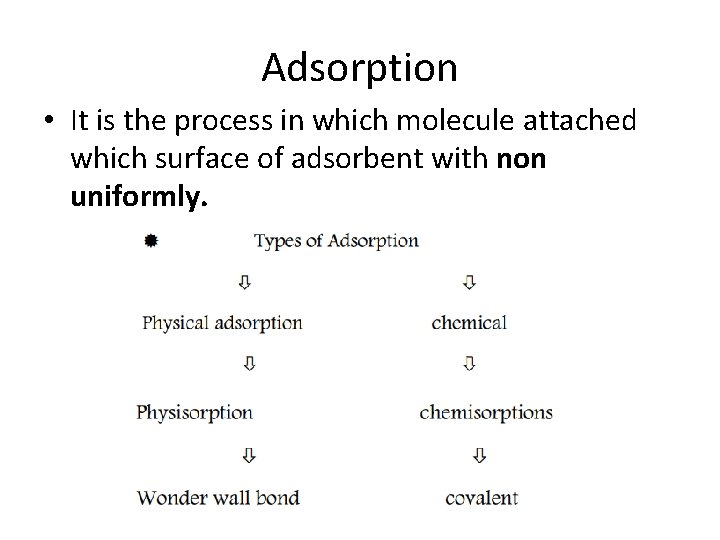
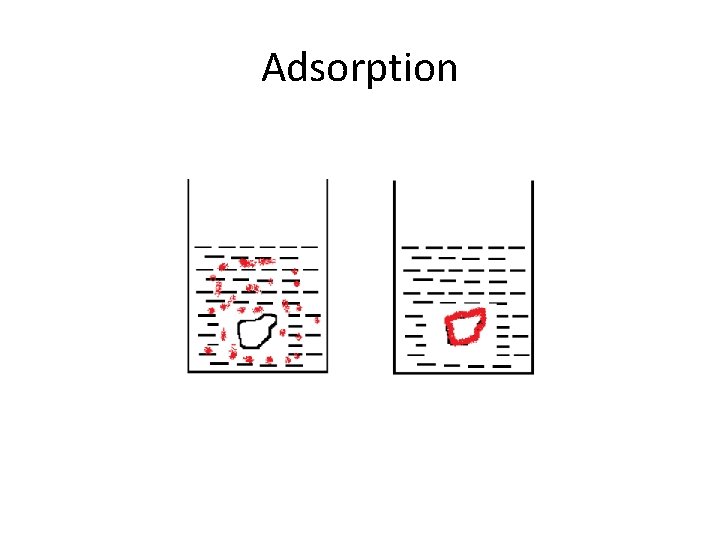
Adsorption • It is the process in which molecule attached which surface of adsorbent with non uniformly.

Adsorption

Adsorption • • Properties: It occurs non uniformly It is surface phenomena It is affected by temperature Application: Textile industry Coating of metal

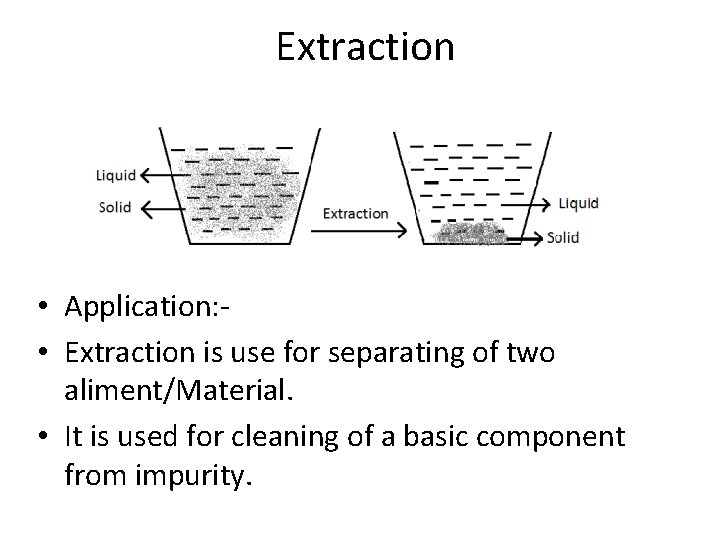
Extraction • Extraction is very common process used when Isolating, purifying a product. • Types of Extraction • Solid- solid Extraction • Liquid- liquid Extraction • Gas- Gas Extraction https: //www. youtube. com/watch? v=KBPv 2 p 7 T 1 wo

Extraction • Application: • Extraction is use for separating of two aliment/Material. • It is used for cleaning of a basic component from impurity.

Thank You