Pressure Conversions 1 atm 1 01325 x 105






- Slides: 17

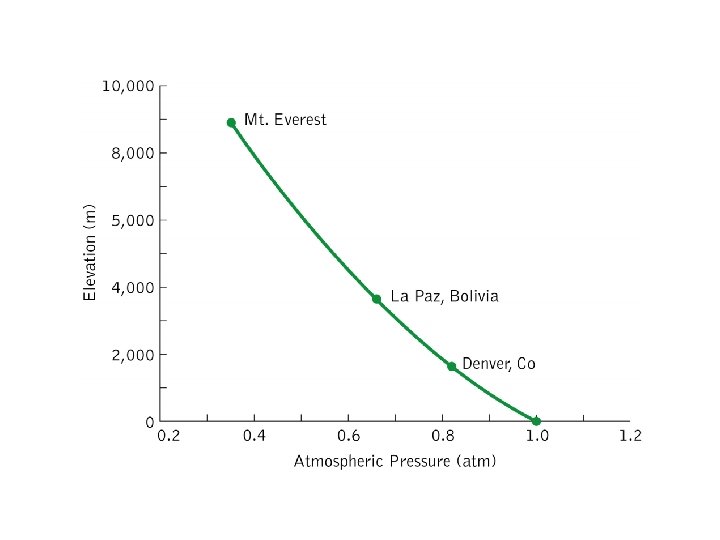
Pressure Conversions • • • 1 atm = 1. 01325 x 105 Pa 1 bar = 1 x 105 Pa 1 millibar (mb) = 100 Pa 1 atm = 1. 01325 bar 1 atm = 760 torr 1 torr = 1 mm Hg



Change volume (V) and the pressure (P) will change (assuming that temperature and the number of molecules are constant) Boyle’s Law PV = constant Pressure and Volume are inversely proportional P 1 V 1 = P 2 V 2

A gas occupying a volume of 725 m. L at a pressure of 0. 970 atm is allowed to expand at constant temperature until its pressure reaches 0. 541 atm. What is the final volume? (725 ml)(0. 970 atm) = (V 2)(0. 541 atm) V 2 = 1300 ml or 1. 30 L

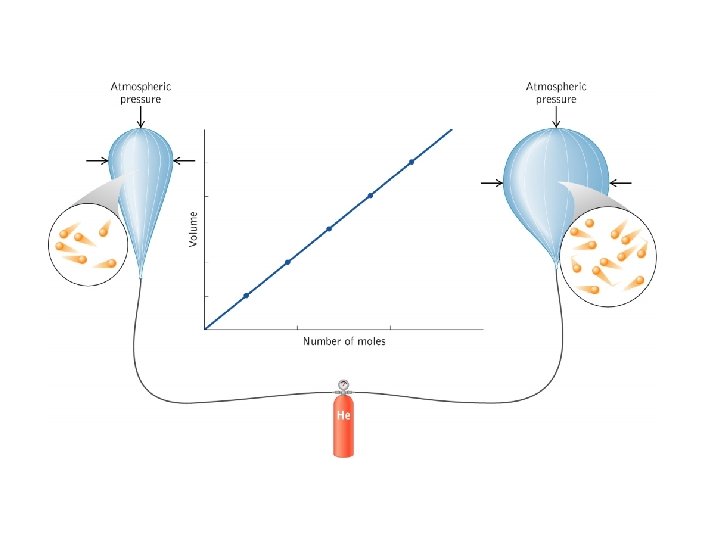
Change the amount of gas (n) and the volume (V) will change (assuming that temperature and pressure constant) Avogadro’s Law n V Number of Moles and Volume are proportional V 1 = V 2 n 1 n 2


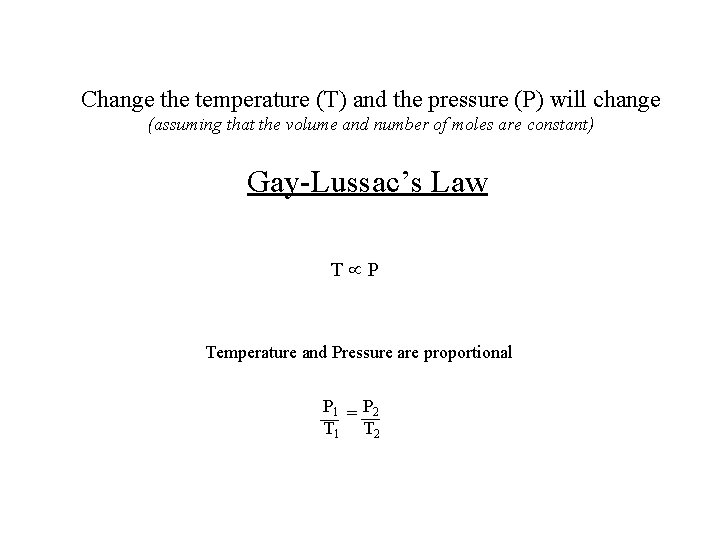
Change the temperature (T) and the pressure (P) will change (assuming that the volume and number of moles are constant) Gay-Lussac’s Law T P Temperature and Pressure are proportional P 1 = P 2 T 1 T 2

An aerosol can is under a pressure of 3. 00 atm at 25 C. Directions on the can caution the user to keep the can in a place where the temperature does not exceed 52 C. What would the pressure of the gas in the aerosol can be at 52 C? 3. 00 atm 298 K = X atm 325 K X = 3. 27 atm

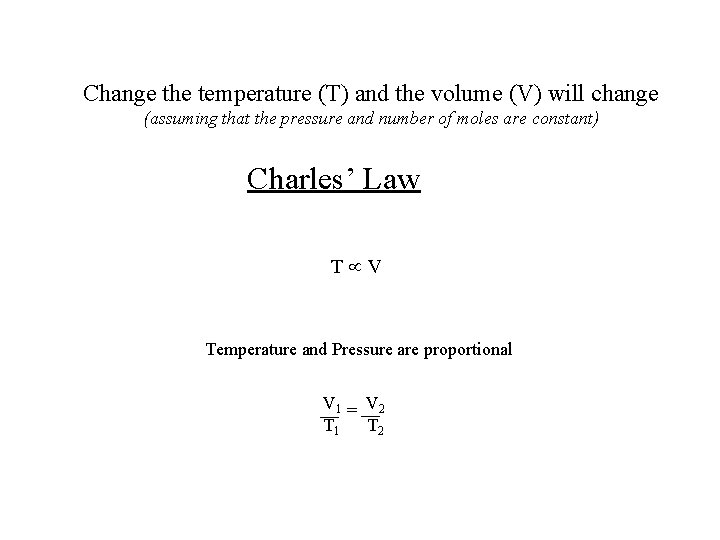
Change the temperature (T) and the volume (V) will change (assuming that the pressure and number of moles are constant) Charles’ Law T V Temperature and Pressure are proportional V 1 = V 2 T 1 T 2

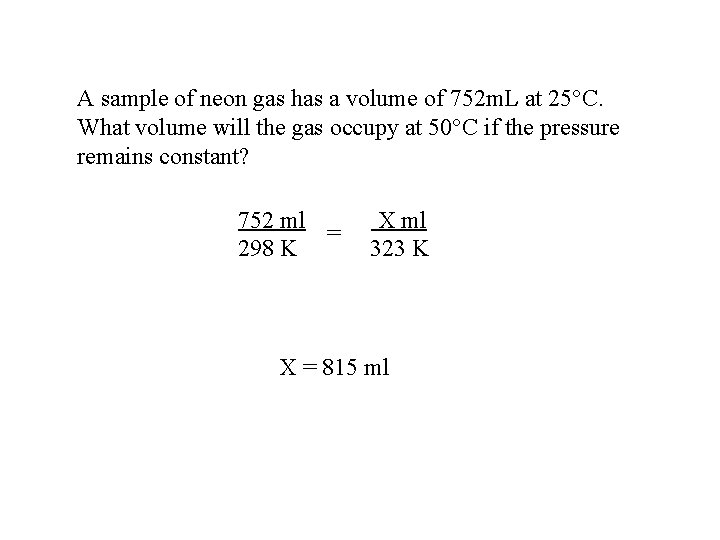
A sample of neon gas has a volume of 752 m. L at 25 C. What volume will the gas occupy at 50 C if the pressure remains constant? 752 ml = 298 K X ml 323 K X = 815 ml

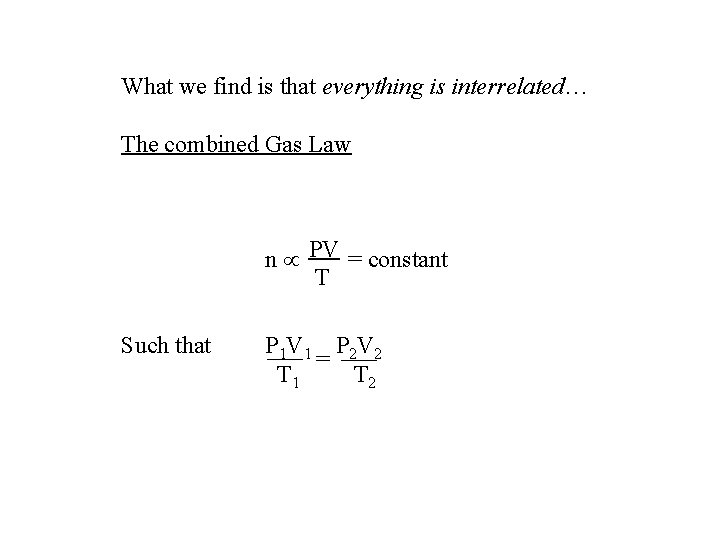
What we find is that everything is interrelated… The combined Gas Law n PV = constant T Such that P 1 V 1 P 2 V 2 = T 1 T 2

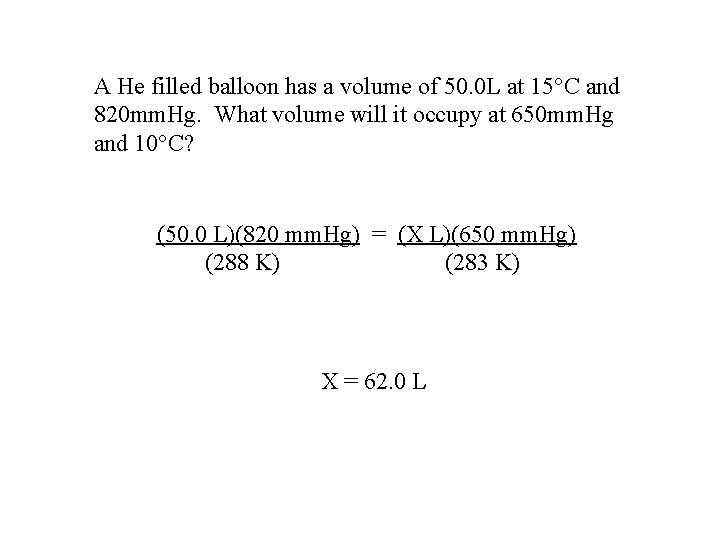
A He filled balloon has a volume of 50. 0 L at 15 C and 820 mm. Hg. What volume will it occupy at 650 mm. Hg and 10 C? (50. 0 L)(820 mm. Hg) = (X L)(650 mm. Hg) (288 K) (283 K) X = 62. 0 L

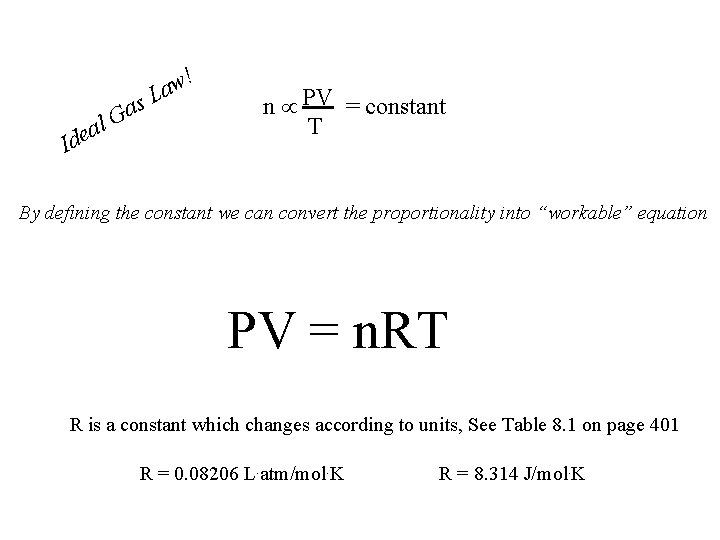
! l a e Id s a G w a L n PV = constant T By defining the constant we can convert the proportionality into “workable” equation PV = n. RT R is a constant which changes according to units, See Table 8. 1 on page 401 R = 0. 08206 L. atm/mol. K R = 8. 314 J/mol. K

Ideal Gas Conditions • • Negligible Interactions Negligible Particle Size High Temperature Low Pressure

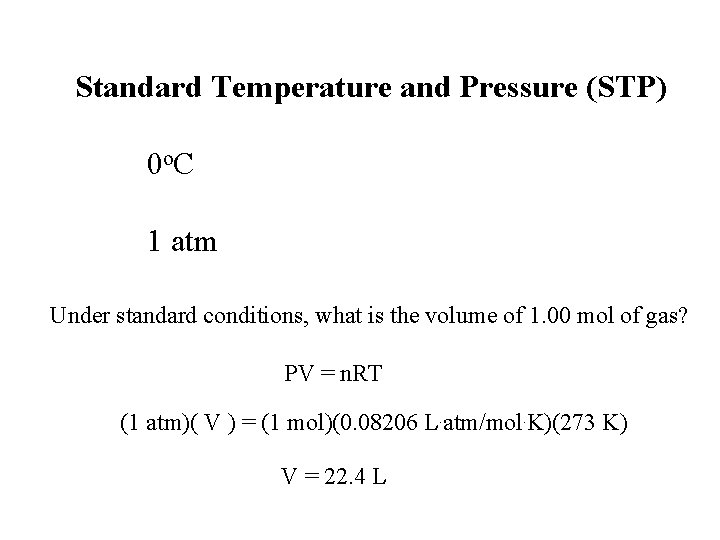
Standard Temperature and Pressure (STP) 0 o. C 1 atm Under standard conditions, what is the volume of 1. 00 mol of gas? PV = n. RT (1 atm)( V ) = (1 mol)(0. 08206 L. atm/mol. K)(273 K) V = 22. 4 L

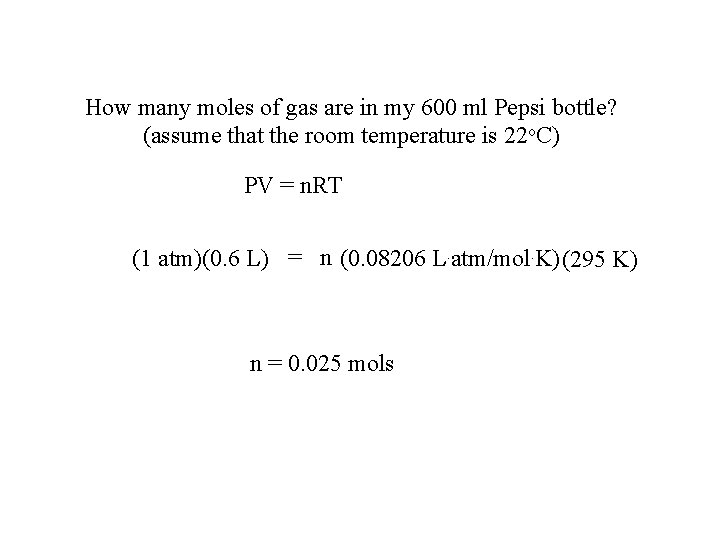
How many moles of gas are in my 600 ml Pepsi bottle? (assume that the room temperature is 22 o. C) PV = n. RT (1 atm)(0. 6 L) = n (0. 08206 L. atm/mol. K)(295 K) n = 0. 025 mols
 Stp formula
Stp formula 1 atm 105 pa
1 atm 105 pa Speed tunnel
Speed tunnel Bevel of et tube
Bevel of et tube Afferent and efferent arterioles
Afferent and efferent arterioles Intrapleural pressure
Intrapleural pressure Blood pressure regulation
Blood pressure regulation Metamorphic
Metamorphic High pressure area
High pressure area Insall salvati ratio
Insall salvati ratio Hydrostatic pressure vs osmotic pressure
Hydrostatic pressure vs osmotic pressure Oncotic pressure vs hydrostatic pressure
Oncotic pressure vs hydrostatic pressure Describing edema
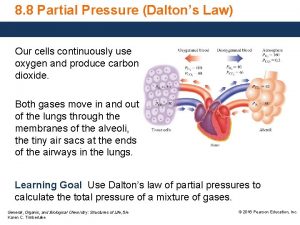
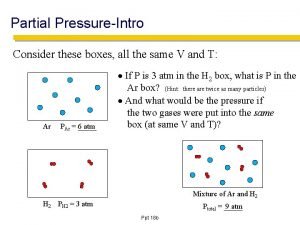
Describing edema How to find partical pressure
How to find partical pressure Sore throat after surgery
Sore throat after surgery Pressure support vs pressure control
Pressure support vs pressure control Oncotic pressure vs hydrostatic pressure
Oncotic pressure vs hydrostatic pressure Hypergraph containers
Hypergraph containers