Planning and Organising a Research Study Anna Miller





































- Slides: 46

Planning and Organising a Research Study Anna Miller, MBCh. B, MPH Senior Technical Advisor EGPAF Global Technical Policy Unit Operations Research Training Kampala, Uganda 5 November 2009

Learning Objectives l Gain a comprehensive understanding of how to plan and organise a study from the literature review stage until utilisation of findings

Session overview 1. Group activity and feedback / discussion 2. Slide presentation 3. Country experience-sharing and Question & Answer
Group Exercise!

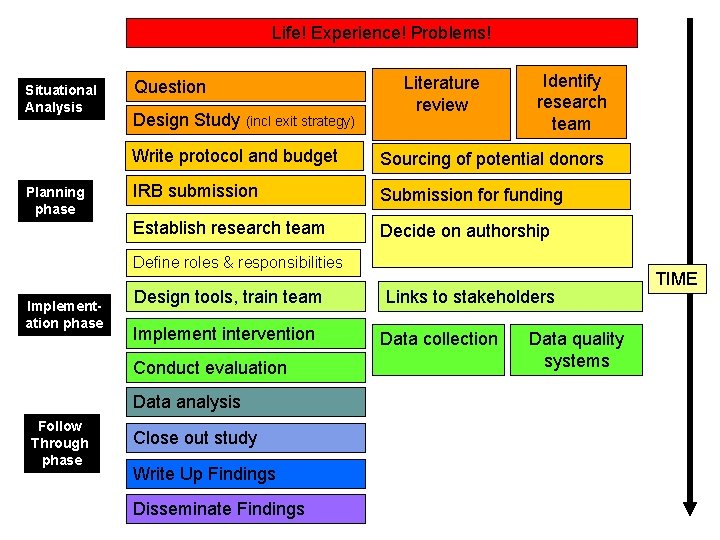
Life! Experience! Problems! Situational Analysis Planning phase Question Design Study (incl exit strategy) Literature review Identify research team Write protocol and budget Sourcing of potential donors IRB submission Submission for funding Establish research team Decide on authorship Define roles & responsibilities Implementation phase Design tools, train team Implement intervention Conduct evaluation Data analysis Follow Through phase Close out study Write Up Findings Disseminate Findings Links to stakeholders Data collection Data quality systems TIME

Life! Experience! Problems! Situational Analysis Question Design Study Planning phase Literature review Identify research team Write protocol and budget Sourcing of potential donors IRB submission Submission for funding Establish research team Decide on authorship Communication Ethical Links to stakeholders Considerations Implement intervention Data collection Data quality Define roles & responsibilities Implementation phase Conduct evaluation Data analysis Close out study Follow Through phase Write Up Findings Disseminate Findings systems

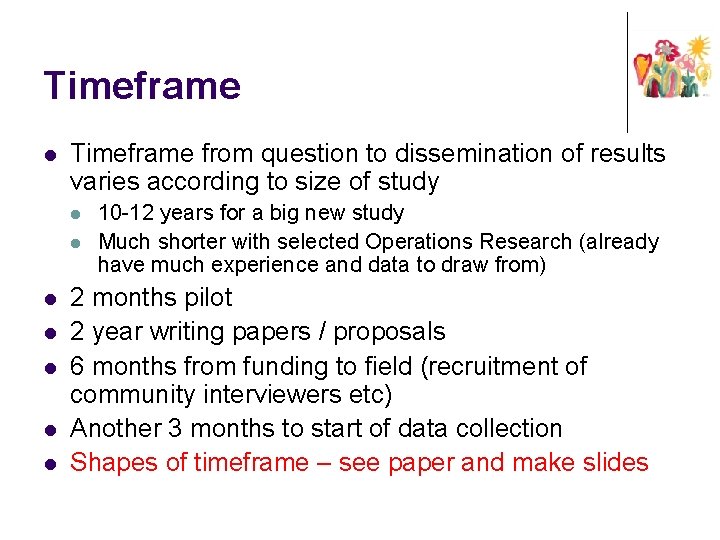
Timeframe l Timeframe from question to dissemination of results varies according to size of study l l l l 10 -12 years for a big new study Much shorter with selected Operations Research (already have much experience and data to draw from) 2 months pilot 2 year writing papers / proposals 6 months from funding to field (recruitment of community interviewers etc) Another 3 months to start of data collection Shapes of timeframe – see paper and make slides

The Seven “P”s l l l l Prior Planning and Preparation Prevents a Pretty Poor Performance

Question & Study Design l l Need to have a good question with the correct methods to answer that question Wrong question + wrong methods = can’t answer question = unethical and waste of resources Involve statistician early and make sure study will sufficiently powered to attribute any changes to the intervention that was implemented Identify your research team early - experts who know about the specific methods and/or issue being investigated

Literature Review l What is a literature review? l Why (and when) are they necessary? l How do I conduct a literature review?

What is a literature review? l A systematic critical review and appraisal of what has been published on a topic by researchers and other experts l Not simply a descriptive account of what has been published l Usually part of a larger piece of work, such as a research paper l Can also be done on its own (e. g. annotated bibliography)

Qualities of a good literature review l Organized around and directly related to the research question that you are addressing l Synthesizes results into a summary of what is and what is not known l Provides the reader with information on the existing knowledge about a topic, and the strengths and weaknesses of that knowledge l Identifies questions for future research

When should I do one? l When writing proposals l When writing research articles l Can you think of any other times when a literature review could be helpful?

Why should I do one? l To identify gaps in current knowledge/practice that merit further investigation (to justify your research) l To demonstrate that your research will fill a gap and contribute to the field l To provide a theoretical/methodological basis for your research l To provide context for your research l To show your audience that you’ve “done your homework”

How to: Conducting literature reviews l What to include l l l Peer-reviewed publications Grey literature Where to search l l Pub. Med (and other databases, e. g. Psyc. Info) Google Scholar Organization / gov’t websites, etc. EGPAF journal access through GW Library l Remember to look in the EGPAF Publication Database (on the intranet) before you request an article… it might already be there!

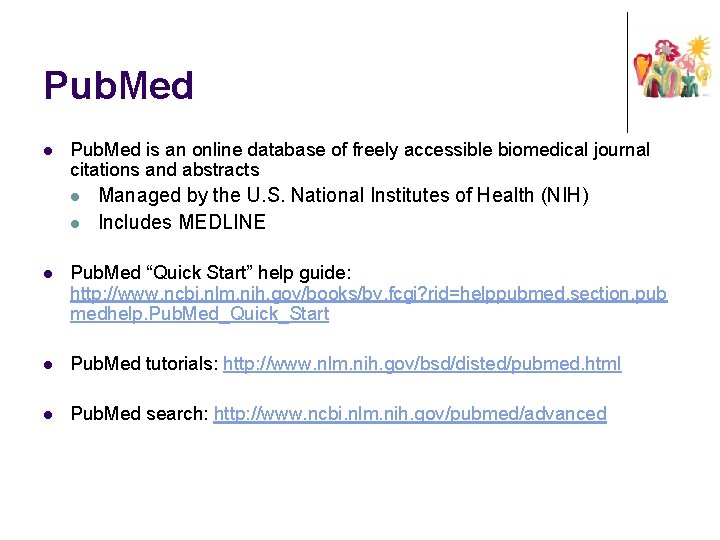
Pub. Med l Pub. Med is an online database of freely accessible biomedical journal citations and abstracts l l Managed by the U. S. National Institutes of Health (NIH) Includes MEDLINE l Pub. Med “Quick Start” help guide: http: //www. ncbi. nlm. nih. gov/books/bv. fcgi? rid=helppubmed. section. pub medhelp. Pub. Med_Quick_Start l Pub. Med tutorials: http: //www. nlm. nih. gov/bsd/disted/pubmed. html l Pub. Med search: http: //www. ncbi. nlm. nih. gov/pubmed/advanced

To do a basic Pub. Med search by subject: 1. Identify the key concepts for your search. For example, if your main question is “What is the risk of HIV transmission through breastfeeding? ” you could enter the terms HIV and breastfeeding in the search box. 2. Enter the terms in the search box. You do not need to use words like “and” or “the. ” 3. Click Search.

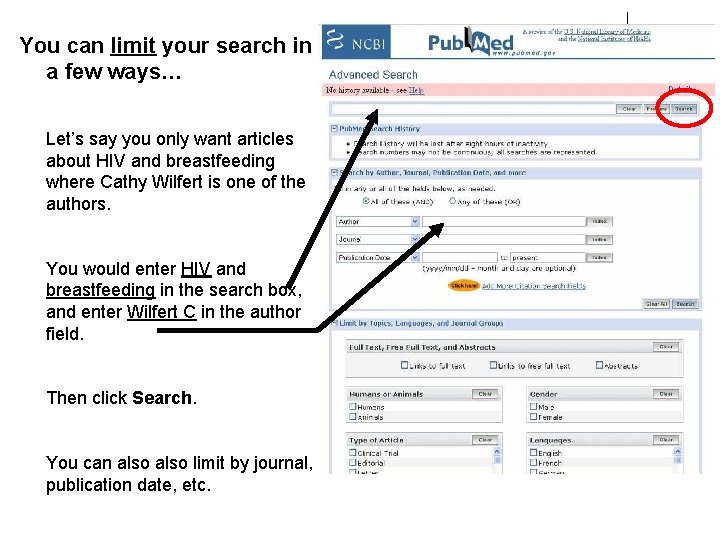
You can limit your search in a few ways… Let’s say you only want articles about HIV and breastfeeding where Cathy Wilfert is one of the authors. You would enter HIV and breastfeeding in the search box, and enter Wilfert C in the author field. Then click Search. You can also limit by journal, publication date, etc.

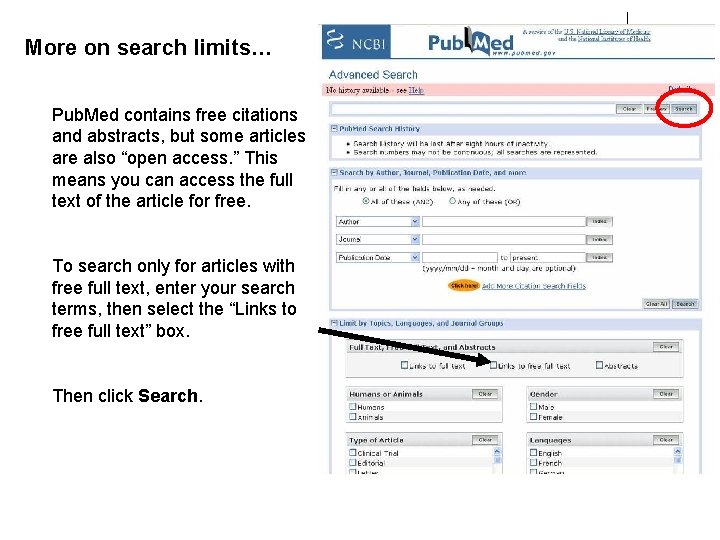
More on search limits… Pub. Med contains free citations and abstracts, but some articles are also “open access. ” This means you can access the full text of the article for free. To search only for articles with free full text, enter your search terms, then select the “Links to free full text” box. Then click Search.

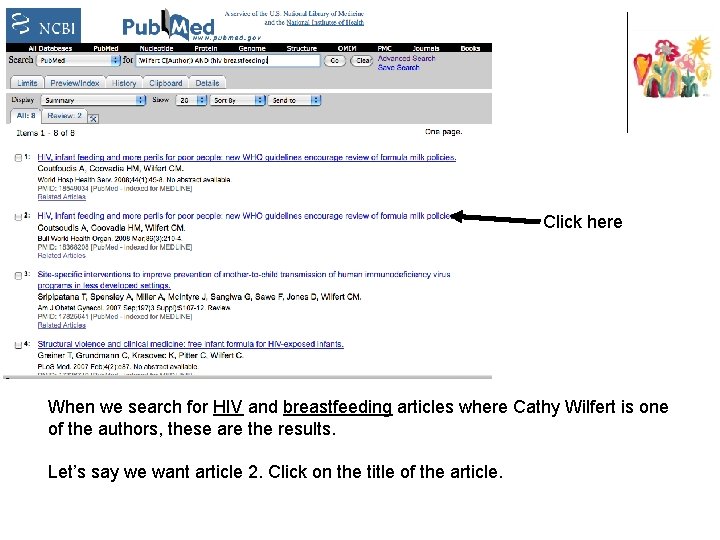
Click here When we search for HIV and breastfeeding articles where Cathy Wilfert is one of the authors, these are the results. Let’s say we want article 2. Click on the title of the article.

Link to free full text This article has a link to free full text. For articles that do not have free full text available, you can request the article through our partnership with GW’s med school library. Instructions for requesting articles through GW library are in your workshop materials.

Google Scholar l Google Scholar allows you to easily search for citations, abstracts, and articles across many disciplines at once l Searches documents from peer-reviewed publications, academic publishers, professional societies, books, etc. l Articles are ranked by weighing the full text of the article, the author, the publication where the article appears, and how often the article is cited in other literature l Google Scholar help: http: //scholar. google. com/intl/en/scholar/help. html l Google Scholar search: http: //scholar. google. com/

You can see the most recent papers by clicking on “Recent articles” 1 2 1. 2. 3. 4. 3 4 Title of the article: This links to the abstract, or full text if available for free. Cited by: Tells you who has cited this paper. Related articles: Searches for similar articles. Web search: Searches the web (via Google) for mentions of this article. As with Pub. Med results, if free full text is not available online, you can request it through our GW library partnership.

Writing a Lit Review: Sections of a literature review l Introduction l l l Body l l Broad overview of your topic Brief explanation of how you organized the literature review (see next slide) Discussion and critical evaluation of the literature Conclusion and recommendations l l l What has the literature taught us? What are the major gaps and why are they important? This section then leads into your research…

Ways to organize your review l “Pro & con”: works that support your position and those that do not l May also consider including works that present an entirely different position l By methodology: Randomized controlled trials, observational studies, etc. l Others: chronological, thematic, trends…

Things to consider when reviewing articles l l l What is the author’s main argument? Is it clearly defined? Has the author evaluated the literature on the topic? Quality and appropriateness of the study design l How was the data collected analyzed? l Were the study components appropriate for the problem? l What are the strengths and limitations of the study? Are the author’s conclusions valid? How do the findings contribute to the field? How does the article and its findings relate to your work specifically?

Exit Strategy For Study l l l Prepare exit strategy at the beginning This is especially important with communities receiving interventions They need to know from the start what they can expect, for how long, and what will happen afterwards. This exit strategy should be integrated into consent, community communications, IEC materials etc It should also include plans for dissemination of results to participating communities

Establish research team l l l Establishing team and setting up systems for management of team (may be combined functions in one or two people depending on size and budget for study. But same principles apply and systems / responsibilities needed for all of these): Finance Administration including procurement Logistics Implementation of intervention Evaluation (should be different from those doing implementation as hard to be objective about the work you’ve done)

Protocol And Budget l l l Need to be written together (as for programming) Protocol requires lead person be identified to do the writing Need to identify funder and use their guidance / formats for protocol and budget Follow submission guidelines for funding and submit by due date! Submit to Institutional Review Board around same time – does not have to be funded before beginning IRB processes (right? !) Factor in sufficient time for regulatory processes – always take longer than you think

Issues For Budget… l l l Produce a time-bound chart of all activities by monthly or quarterly basis. Cost each activity Often divided into in and out of country costs (international collaborations) Budget lines may include: l l l l l Supplies and equipment Office Research (intervention, data management and analysis) Administration Salaries Consultants Communication with community advisory boards Regulatory fees Participant reimbursement as per national standards (e. g. 150 R per visit in SA; 3 -5 USd per visit in Zim)

Establish Research Team l l l Identify person in charge! Document specific roles and responsibilities of individuals and institutions involved Agree any ongoing involvement if current employment arrangements change Involve statistician / data analyst at design stage; don’t wait until the end Outline your data analysis plan at the time of funding and certainly before data collection starts

Authorship Definition of an Author 1. Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; 2. drafting the article or revising it critically for important intellectual content; and 3. final approval of the version to be published. Authors should meet conditions 1, 2, and 3. Adapted from: International Committee of Medical Journal Editors www. icmje. org

Contributorship Examples of Contributors 1. Person who provided purely technical help and/or writing assistance 2. Department chair or supervisor who provided only general support All contributors who do not meet the criteria for authorship should be listed in an acknowledgments section Adapted from: International Committee of Medical Journal Editors www. icmje. org

Design Tools & Train Team l l l Develop appropriate research tools to fit the methods and answer the question Develop written protocols for all aspects of research, data collection and quality assurance of data Involve statistician at design stage of tools Develop locally appropriate IEC materials and informed consent forms Train everyone involved in research team in specific protocols and tools

Linkages to others including policy makers and communities l l l Programmes made stronger by evidence and research made stronger by understanding programme needs and realities Stakeholders can also give valuable input to improve the research approach Make early linkages with policy makers and key stakeholders who will take the findings of the study forwards into action They will be more likely to do this if they are aware and invested from the beginning Community advisory boards

Implement intervention and conduct evaluation as per protocol …

Data Collection & Quality l l l Serious offence to fabricate data Get people invested in good data quality from the outset Early involvement of statistician and data analysis plan Written protocol for data collection, quality assurance and analysis Collect data meticulously Make sure: l l Good quality questions Interviewers appropriately trained Right conditions for delivery of questions (e. g. being overheard asking confidential questions) Additional quality control including blind retesting of samples

Data Analysis l l l Have written data analysis plan matching variables with questions being asked Develop this with statistician right at the beginning Refine it as needed during implementation and data collection phase Data analysis is an iterative process between all investigators (including statistician) – not a “one stop shop” See example data analysis plan

Writing Up Findings l Prioritise writing up informally to share with community participants and local stakeholders as early as possible l l l Often forgotten Can offer helpful interpretations and discussion Plan all publications, their authors and timeframe for completion, submission Generally use IMRAD Format – see writing workshop and other resources Follow guidelines for authorship Select target journal and use their guidelines for submission

Overlapping Publications l l Duplicate submission: most journals do not accept manuscripts being considered simultaneously by other journals Redundant publication: publication of a paper that overlaps substantially with one already published in print or electronic media Adapted from: International Committee of Medical Journal Editors www. icmje. org

Potential Conflicts of Interest: Project Support l l The conditions of project funding have the potential to bias and otherwise discredit the research. Authors should describe the role of the study sponsor, if any, in: l l l study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication. If the supporting source had no such involvement, the authors should so state. Adapted from: International Committee of Medical Journal Editors www. icmje. org

Privacy and Confidentiality: Patients and Study Participants l l l Identifying information should not be published unless it is essential for scientific purposes In these cases, the patient (or parent/guardian) must give written informed consent for publication Informed consent should be obtained if there is any doubt that anonymity can be maintained. l For example, masking the eye region in photographs of patients is inadequate protection of anonymity. Adapted from: International Committee of Medical Journal Editors www. icmje. org

Think You’re Finished? Think Again! Most documents will have to go through some or all of the following processes before they are ready to be distributed to the public: l Author and co-author approval l Partner/donor approval l Permissions for data use and/or borrowed material l Copyediting and Proofreading For peer-reviewed l Design and Typesetting articles, publisher will handle these steps l Printing l Dissemination

Share country experiences Q&A

Questions For Discussion (if needed) l l What are your own experiences in beginning a piece of research and taking it to its conclusion? How did the sequence of events unfold and what did you learn from this? If you were starting to answer a new operations research question yourself tomorrow, what would you differently? Have you experienced any challenges regarding authorship or conflict of interest? If so, how were these resolved? Adapted from: International Committee of Medical Journal Editors www. icmje. org

Acknowledgments l l Frances Cowan Jo Keatinge Cori Mazzeo Sara Teitelman
 What is a project schedule
What is a project schedule Planning skills in the workplace
Planning skills in the workplace Organizing staffing directing controlling
Organizing staffing directing controlling Staffing and directing
Staffing and directing Concept of organising
Concept of organising Lambini and sons
Lambini and sons Self organising map
Self organising map Organizing seminars and conferences
Organizing seminars and conferences Miller indices plane passes through origin
Miller indices plane passes through origin Dbu writing center
Dbu writing center Medical plaza miller 131 miller street
Medical plaza miller 131 miller street Anna miller's menu
Anna miller's menu Miller's magical number 7 study
Miller's magical number 7 study Centre for research anna university
Centre for research anna university N planning
N planning Long term plan and short term plan
Long term plan and short term plan Language policy and planning slideshare
Language policy and planning slideshare Process of method study
Process of method study Objective of work study
Objective of work study Differentiate between time study and motion study
Differentiate between time study and motion study Long term and short term financial planning
Long term and short term financial planning Scope of the study
Scope of the study Strategic planning vs tactical planning
Strategic planning vs tactical planning Goal achievement matrix
Goal achievement matrix Scenario planning workforce planning
Scenario planning workforce planning Perencanaan agregat ppt
Perencanaan agregat ppt Aggregate planning is capacity planning for
Aggregate planning is capacity planning for Examples of aggregate planning
Examples of aggregate planning Planning meals chapter 10 study sheet
Planning meals chapter 10 study sheet What is basic research
What is basic research Contrast applied research and basic research
Contrast applied research and basic research Longitudinal research and cross sectional research
Longitudinal research and cross sectional research Example of applied research
Example of applied research Types of conclusive research design
Types of conclusive research design What is inquiry in practical research
What is inquiry in practical research The meaning of
The meaning of What is case series
What is case series Retrospective cohort study
Retrospective cohort study Study less study smart
Study less study smart Phytogeographical regions of india
Phytogeographical regions of india Research process
Research process Enterprise research planning
Enterprise research planning Action research vs case study
Action research vs case study General objective in research
General objective in research Different types of research designs
Different types of research designs Locale of the study
Locale of the study Why choose case study research design
Why choose case study research design