Physical Properties of Matter Mass versus Weight Although



















- Slides: 20

Physical Properties of Matter

Mass versus Weight • Although the terms mass and weight are used • • almost interchangeably, there is a difference between them. Mass is a measure of the quantity of matter, which is constant all over the universe. Weight is proportional to mass but depends on location in the universe. Weight is the force exerted on a body by gravitational attraction (usually by the earth).

Example • The mass of a man is constant. However the man may weigh: 150 lbs on earth, 25 lbs on the moon (because the force of gravity on the moon is 1/6 that of the earth), and be "weightless" in space.

Intrinsic Properties • do not depend on the amount of the sample • being examined. Examples include – – – Color Boiling Point Melting Point Density Odor • They cannot be changed without changing the object itself.

Extensive Properties • depend on the quantity of the sample • Examples: – Mass – Volume • The object itself does not change if an extrinsic property changes.

Two Types of Properties • Chemical Properties: Properties that are based on how a substance chemically reacts with other substances. • Physical Properties: Can be observed or measured without changing the composition of matter. Physical properties are used to observe and describe matter.

Physical Properties • Physical properties include: – – – – Appearance Texture Color Odor melting point and boiling point Density Solubility and polarity and many others.

States of Matter • Five basic states: – Solids – Liquids – Gases – Plasmas – Bose-Einstein

Solids • A substance in a solid phase is relatively rigid, • • has a definite volume and shape. The atoms or molecules that comprise a solid are packed close together and are not compressible. Because all solids have some thermal energy, its atoms do vibrate. However, this movement is very small and very rapid, and cannot be observed under ordinary conditions.

Liquids • Liquids have a definite volume, but are able to • • • change their shape by flowing. Liquids are similar to solids in that the particles touch. However the particles are able to move around. Since particles are able to touch the densities of liquid will be close to that of a solid. Since the liquid molecules can move they will take the shape of their container.

More on Liquids • Viscosity --The resistance of a liquid to flow is called its • • • viscosity Surface Tension -- The result of attraction between molecules of a liquid which causes the surface of the liquid to act as a thin elastic film under tension. Surface tension causes water to form spherical drops or a Menicus. Vapor Pressure -- The pressure that a solid or liquid exerts when it is in equilibrium with its vapor at a given temperature. Boiling Point -- when vapor pressure = atmospheric pressure. (it turns into a gas)

Gases • Gases have no definite volume or shape. If unconstrained gases will spread out indefinitely. If confined they will take the shape of their container. • This is because gas particle have enough energy to overcome attractive forces. • Each of the particles are well separated resulting in a very low density.

Plasma • Plasma is an ionized gas, a gas into which sufficient energy is provided to free electrons from atoms or molecules and to allow both species, ions and electrons, to coexist. In effect a plasma is a cloud of protons, neutrons and electrons where all the electrons have come loose from their respective molecules and atoms, giving the plasma the ability to act as a whole rather than as a bunch of atoms.

More on Plasma • Plasmas are the most common state of matter in the universe comprising more than 99% of our visible universe and most of that not visible. • Plasma occurs naturally and makes up the stuff of our sun, the core of stars and occurs in quasars, x-ray beam emitting pulsars, and supernovas.

Even More on Plasma • On earth, plasma is naturally occurring in flames, lightning and the auroras.

And now a fifth state -- Bose Einstein? • Recently, scientists have discovered the Bose • Einstein condensate, which can be thought of as the opposite of a plasma. It occurs at ultra-low temperature, close to the point that the atoms are not moving at all. A Bose-Einstein condensate is a gaseous superfluid phase formed by atoms cooled to temperatures very near to absolute zero.

Density • Density of Solids and Liquids is determined by taking the mass in Grams and dividing by the Volume in m. L or cm 3 • Density of Gases is determined by taking the mass in Grams and dividing by the volume in L

More on Density • Density defined in a qualitative manner as the • • measure of the relative "heaviness" of objects with a constant volume. a physical property of matter, as each element and compound has a unique density associated with it. If we determine the density, we can compare it to a known list and determine the substance.

Even More on Density • Objects that are less dense float. • Objects that are more dense sink. • Water has a density of 1. 0 g/m. L. • Objects less dense will float • Objects more dense will sink

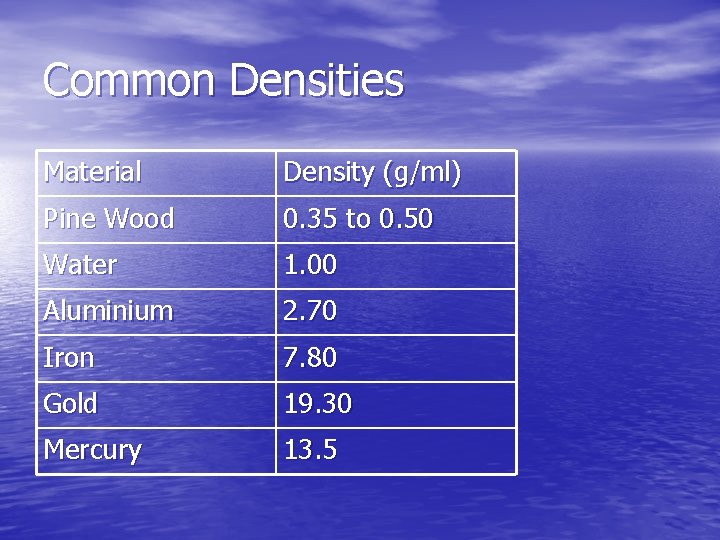
Common Densities Material Density (g/ml) Pine Wood 0. 35 to 0. 50 Water 1. 00 Aluminium 2. 70 Iron 7. 80 Gold 19. 30 Mercury 13. 5
 Mass and weight for kids
Mass and weight for kids Mass versus weight
Mass versus weight Why is mass more useful than weight for measuring matter
Why is mass more useful than weight for measuring matter Physical properties of matter jeopardy
Physical properties of matter jeopardy Graphic organizer of matter
Graphic organizer of matter Physical properties of notebook paper
Physical properties of notebook paper Chemical property definition
Chemical property definition Tolerable weight is a body weight
Tolerable weight is a body weight Weight reducing industry
Weight reducing industry Logical memory vs physical memory
Logical memory vs physical memory Spare change physical versus chemical change
Spare change physical versus chemical change Matter vs weight
Matter vs weight Volume * density = mass
Volume * density = mass Mass vs wieght
Mass vs wieght Weight equals mass times gravity
Weight equals mass times gravity Mass vs weight venn diagram
Mass vs weight venn diagram Mass vs weight venn diagram
Mass vs weight venn diagram Newtons st law
Newtons st law Difference between weight and mass
Difference between weight and mass How is mass different from weight? *
How is mass different from weight? * How is mass different from weight? *
How is mass different from weight? *