Notes One Unit Three Define Chemistry Mass and

























- Slides: 73

Notes One Unit Three Define Chemistry Mass and Volume Density Weight versus Mass Atom Versus Element Atoms, Elements, Molecules Reading Scales Pages 308 -317

What is Chemistry? Chemistry is the study of matter and the changes it undergoes

Chemistry is……Cooking!

Chemistry is. . . Forensics

Chemistry Is. . . Industry

Chemistry is……Art

Chemistry is everything!!!!!!

• • There are three states of matter. What are they? Solid Liquid gas

What are chemical Reactions?

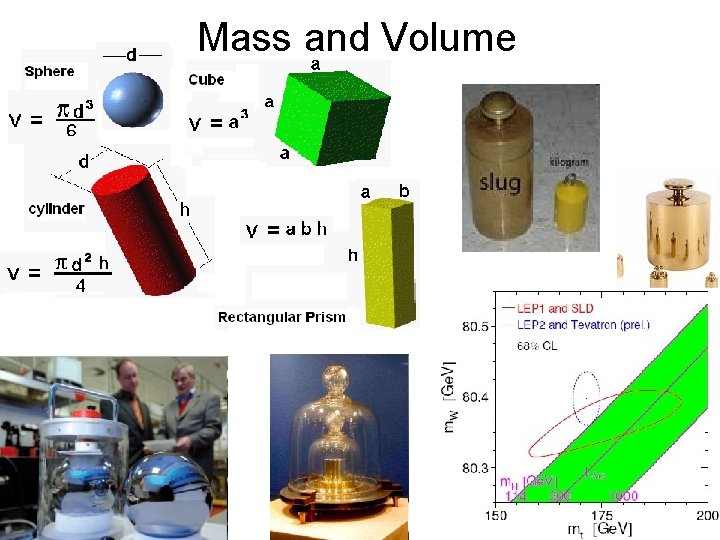
Mass and Volume

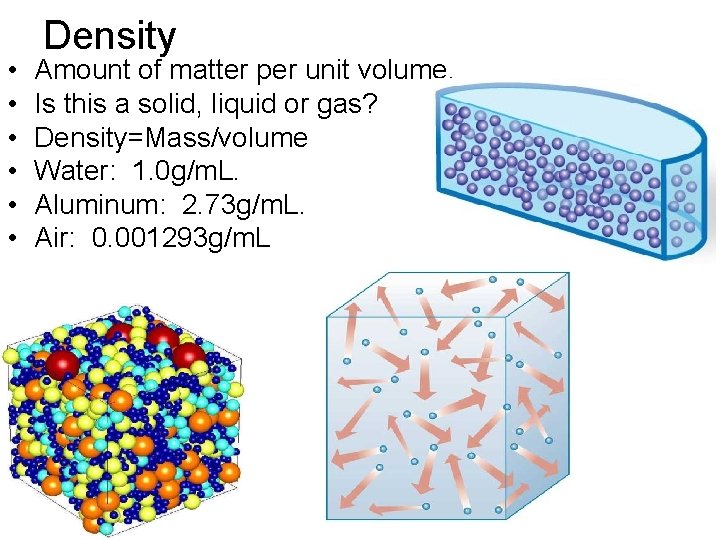
• • • Density Amount of matter per unit volume. Is this a solid, liquid or gas? Density=Mass/volume Water: 1. 0 g/m. L. Aluminum: 2. 73 g/m. L. Air: 0. 001293 g/m. L

Why is density important?

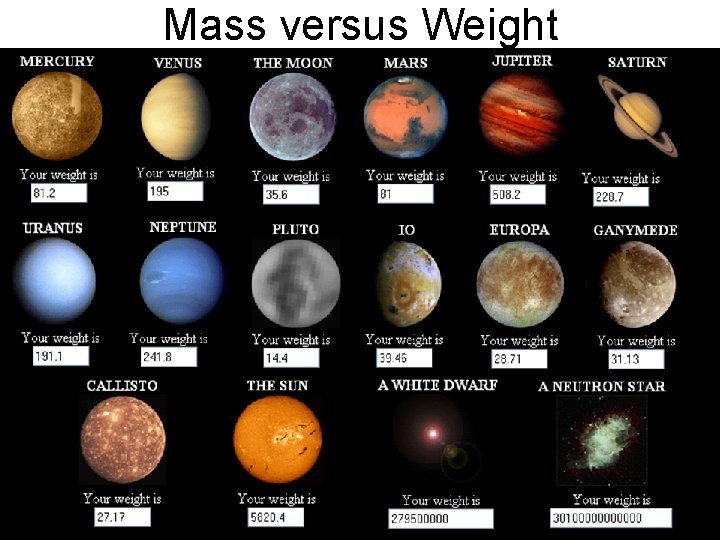
• • • Weight versus Mass VS Weight mass matter weight force 1 lb feathers and 1 lb lead? more weight? more mass?

Mass versus Weight

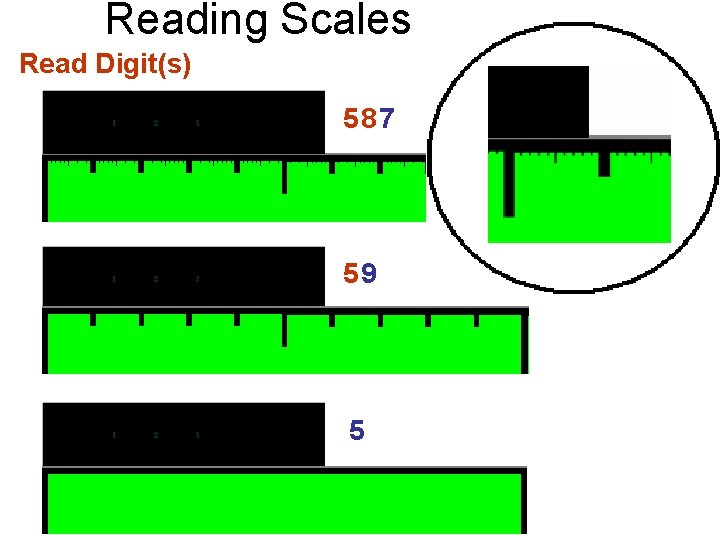
Reading Scales Read Digit(s) Estimated Digit 587 59 5

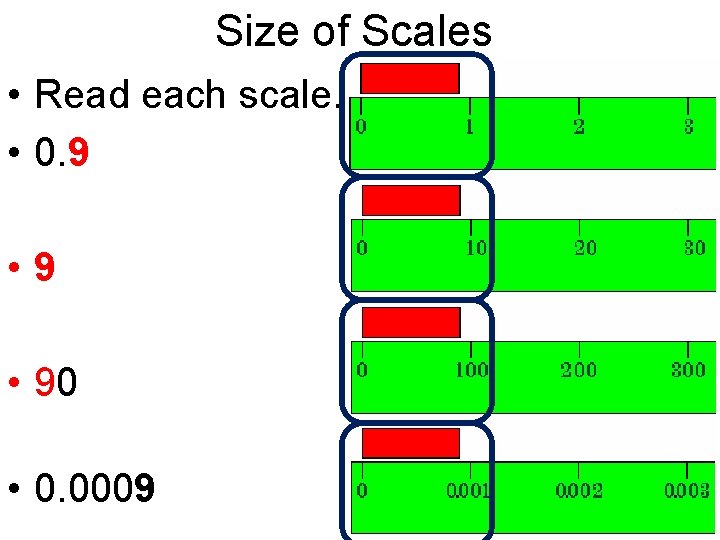
Size of Scales • Read each scale. • 0. 9 • 90 • 0. 0009

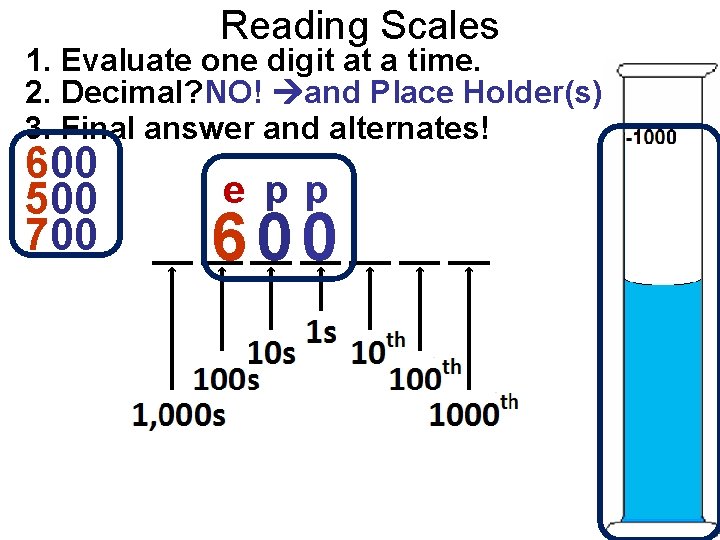
Reading Scales 1. Evaluate one digit at a time. 2. Decimal? NO! and Place Holder(s)! 3. Final answer and alternates! 600 500 700 e pp 600

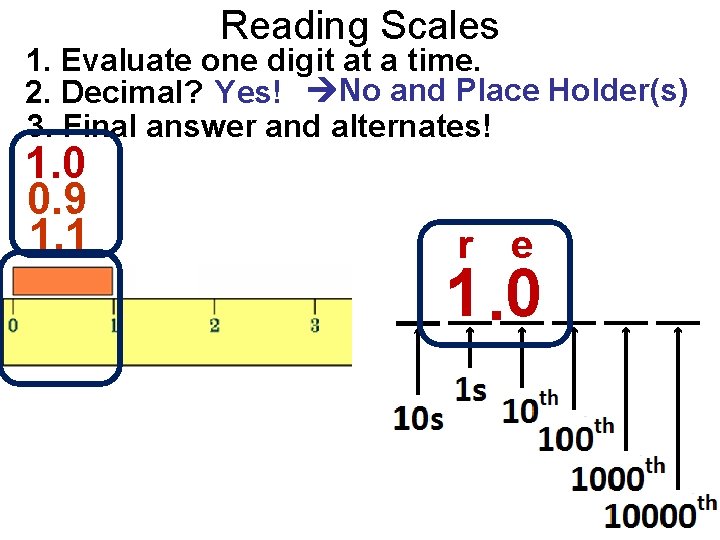
Reading Scales 1. Evaluate one digit at a time. 2. Decimal? Yes! No and Place Holder(s) 3. Final answer and alternates! 1. 0 0. 9 1. 1 r e 1. 0

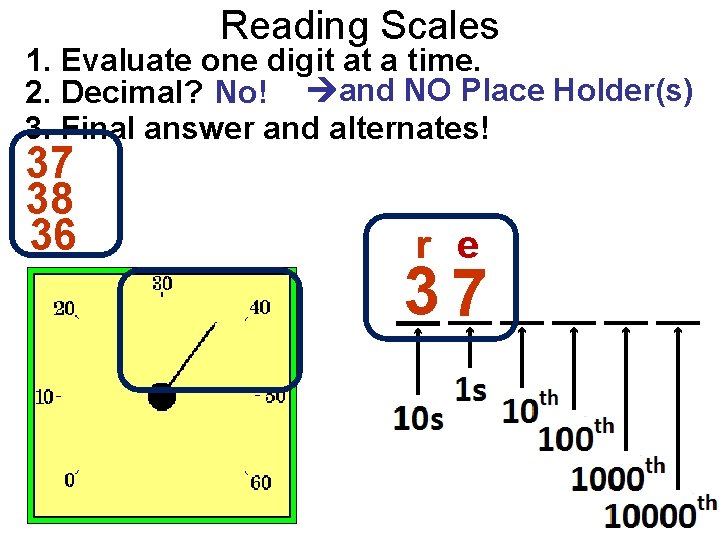
Reading Scales 1. Evaluate one digit at a time. 2. Decimal? No! and NO Place Holder(s) 3. Final answer and alternates! 37 38 36 r e 37

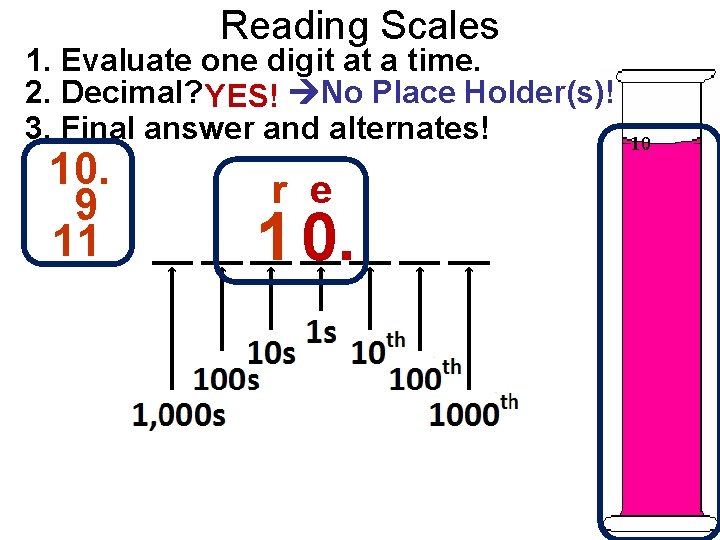
Reading Scales 1. Evaluate one digit at a time. 2. Decimal? YES! No Place Holder(s)! 3. Final answer and alternates! 10. 9 11 r e 1 0.

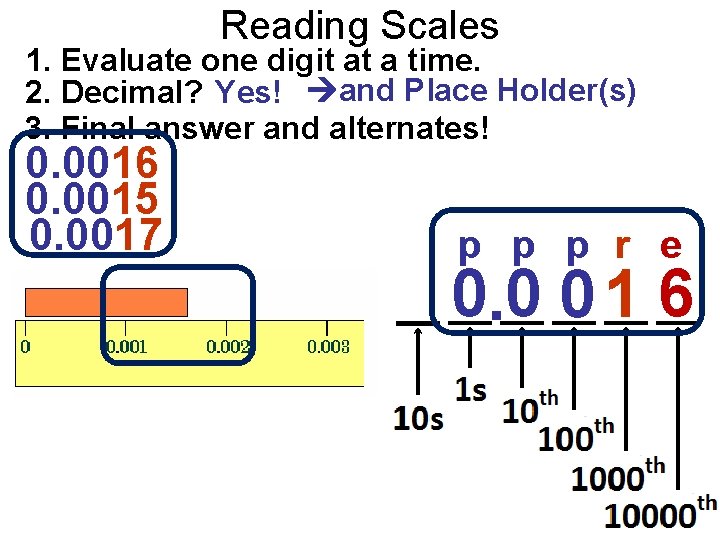
Reading Scales 1. Evaluate one digit at a time. 2. Decimal? Yes! and Place Holder(s) 3. Final answer and alternates! 0. 0016 0. 0015 0. 0017 p p p r e 0. 0 0 1 6

End of Try One

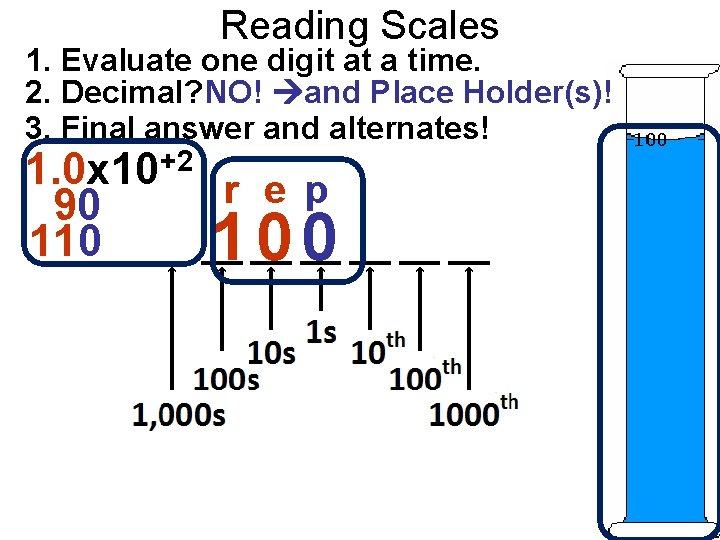
Reading Scales 1. Evaluate one digit at a time. 2. Decimal? NO! and Place Holder(s)! 3. Final answer and alternates! 1. 0 x 10+2 r e p 90 110 100

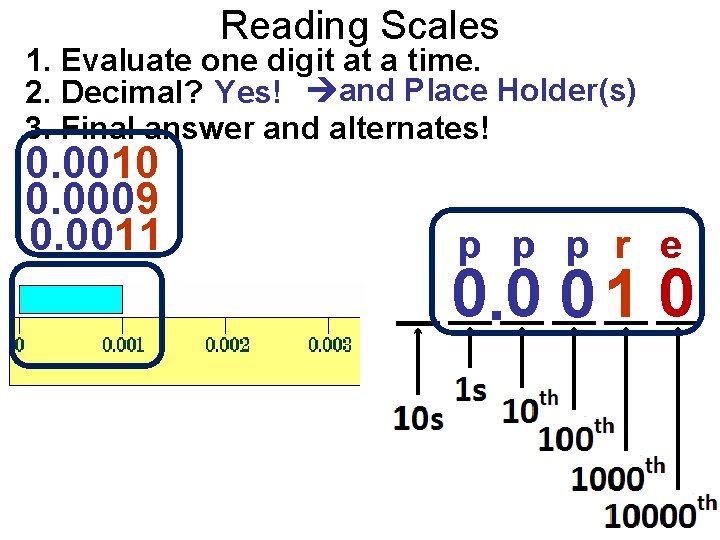
Reading Scales 1. Evaluate one digit at a time. 2. Decimal? Yes! and Place Holder(s) 3. Final answer and alternates! 0. 0010 0. 0009 0. 0011 p p p r e 0. 0 0 1 0

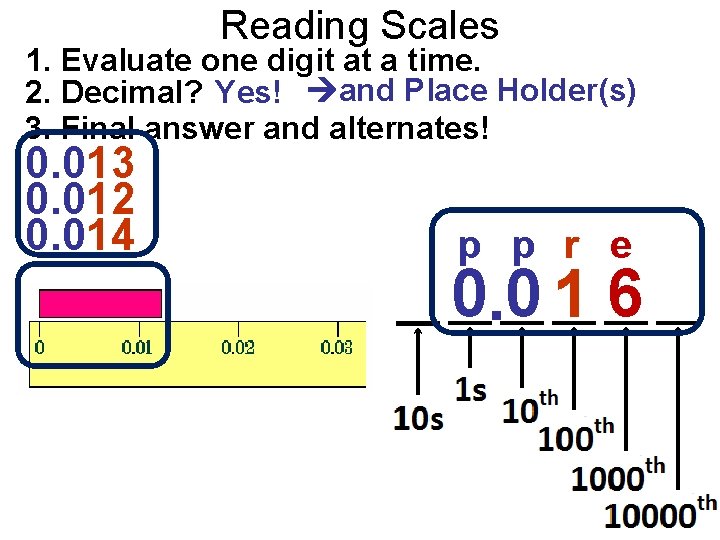
Reading Scales 1. Evaluate one digit at a time. 2. Decimal? Yes! and Place Holder(s) 3. Final answer and alternates! 0. 013 0. 012 0. 014 p p r e 0. 0 1 6

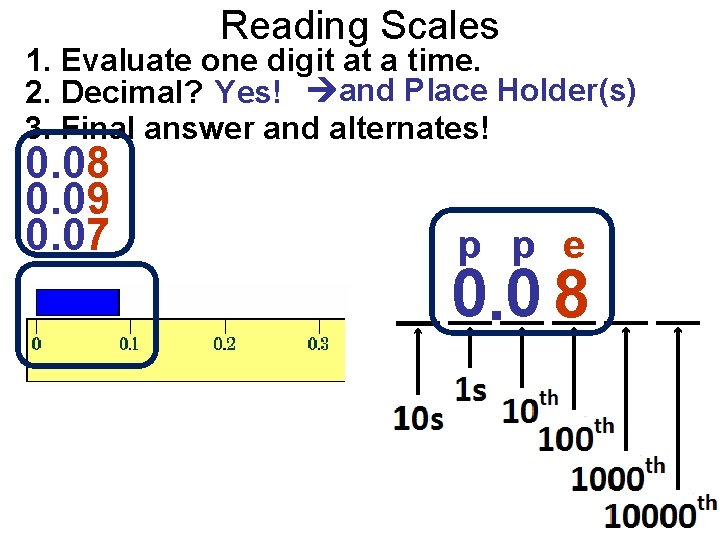
Reading Scales 1. Evaluate one digit at a time. 2. Decimal? Yes! and Place Holder(s) 3. Final answer and alternates! 0. 08 0. 09 0. 07 p p e 0. 0 8

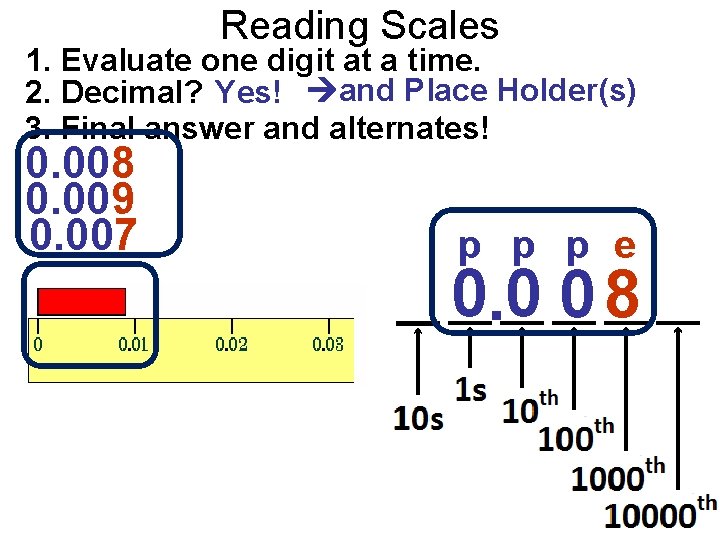
Reading Scales 1. Evaluate one digit at a time. 2. Decimal? Yes! and Place Holder(s) 3. Final answer and alternates! 0. 008 0. 009 0. 007 p p p e 0. 0 0 8

Notes Two Unit Three • • Quiz Reading Scales/Review Check The Importance of Measurement Accuracy and Precision Scientific Notation Significant Digit Rules Addition and Subtraction Computer Assignment Number Two Pages 56 -64

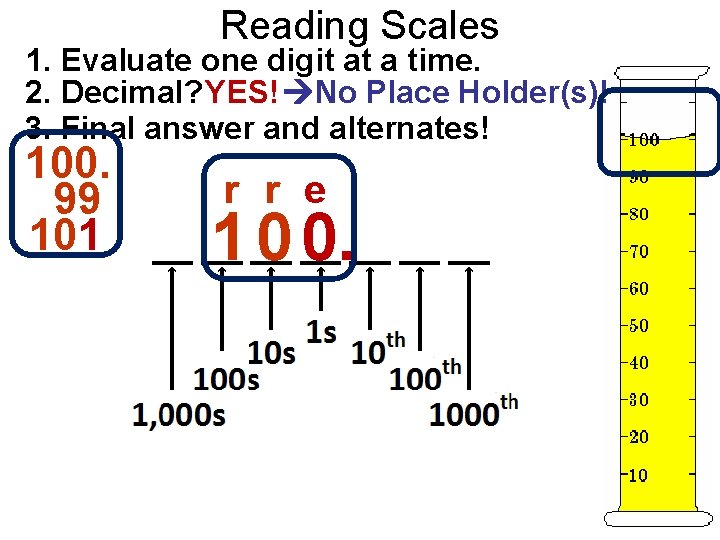
Reading Scales 1. Evaluate one digit at a time. 2. Decimal? YES! No Place Holder(s)! 3. Final answer and alternates! 100. 99 101 r r e 1 0 0.

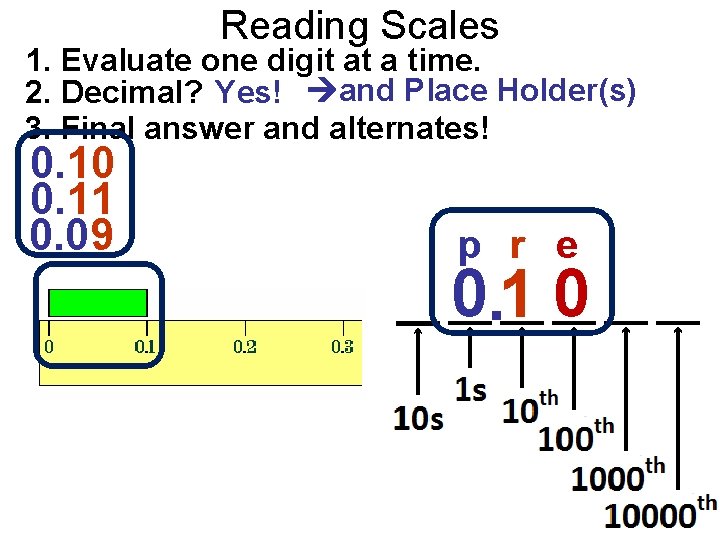
Reading Scales 1. Evaluate one digit at a time. 2. Decimal? Yes! and Place Holder(s) 3. Final answer and alternates! 0. 10 0. 11 0. 09 p r e 0. 1 0

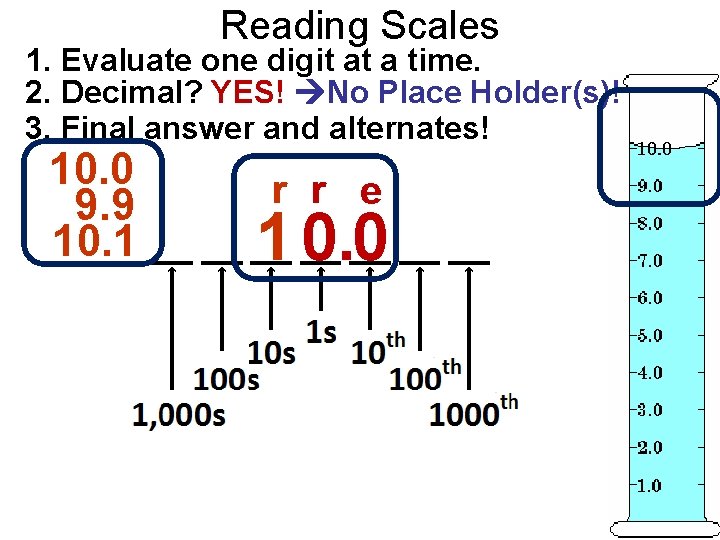
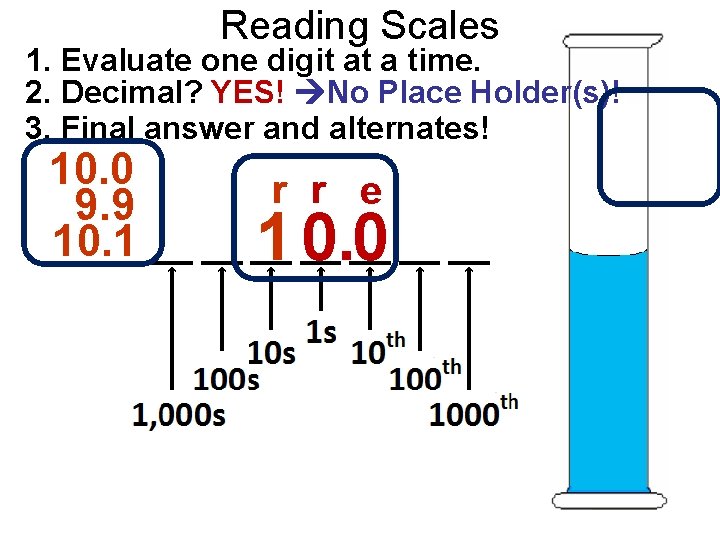
Reading Scales 1. Evaluate one digit at a time. 2. Decimal? YES! No Place Holder(s)! 3. Final answer and alternates! 10. 0 9. 9 10. 1 r r e 1 0. 0

Seven Rows Eight Rows

The Importance of Measurement • • Qualitative measurements (OBSERVATIONS) Quantitative measurements (SCALES) • • • red 12. 0 g/mol liquid 2 eggs 1 dozen 4 legs 1. 0 g/m. L 25 years old 250 pounds Qualitative Quantitative Qualitative Quantitative

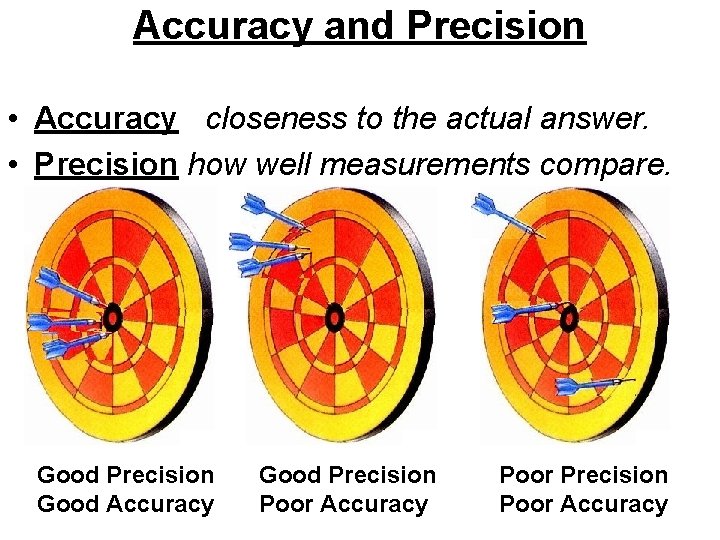
Accuracy and Precision • Accuracy closeness to the actual answer. • Precision how well measurements compare. Good Precision Good Accuracy Good Precision Poor Accuracy Poor Precision Poor Accuracy

Scientific Notation 41200 is… 4. 12 x 10+4 0. 0400 is… 4. 00 x 10 -2 301 is… 3. 01 x 10+2 0. 00560 is… 5. 60 x 10 -3 4. 12 E+4 4. 00 E-2 3. 01 E+2 5. 60 E-3

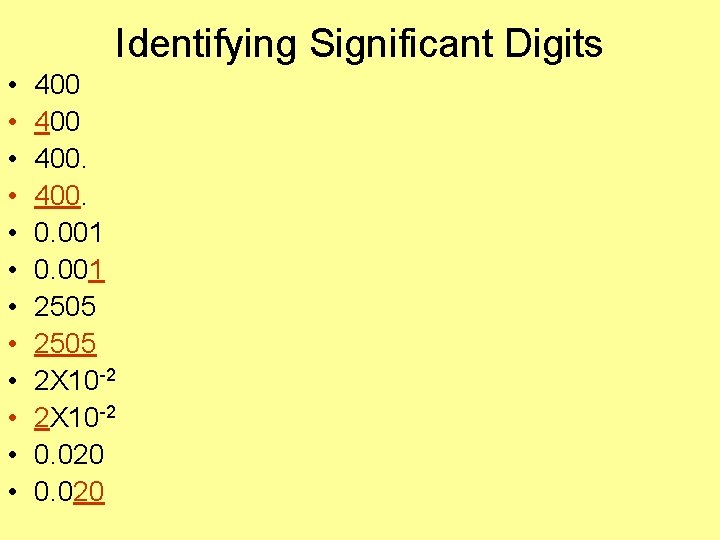
Identifying Significant Digits • • • 400 400. 0. 001 2505 2 X 10 -2 0. 020

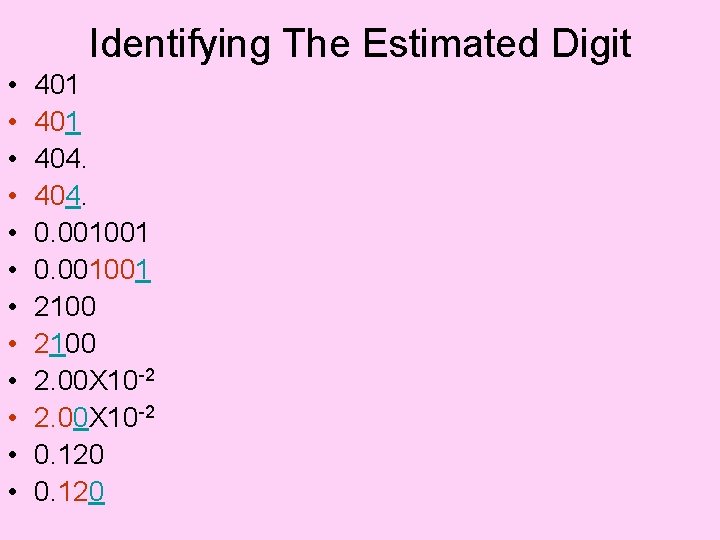
Identifying Significant Digits • • • 401 404. 0. 001001 2100 2. 00 X 10 -2 0. 120

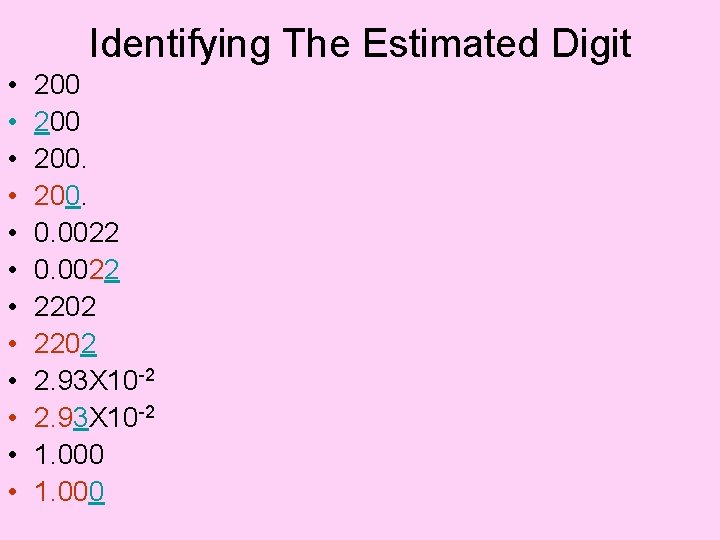
Identifying Significant Digits • • • 200 200. 0. 00220 2202 2. 93 X 10 -2 1. 000

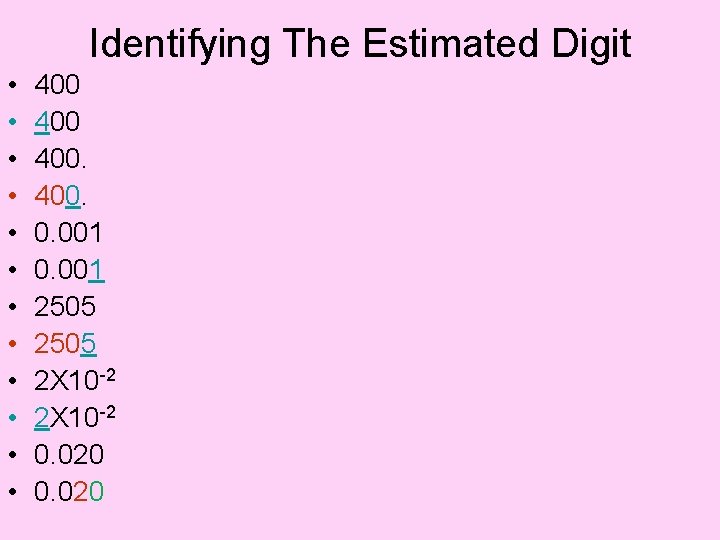
Identifying The Estimated Digit • • • 400 400. 0. 001 2505 2 X 10 -2 0. 020

Identifying The Estimated Digit • • • 401 404. 0. 001001 2100 2. 00 X 10 -2 0. 120

Identifying The Estimated Digit • • • 200 200. 0. 0022 2202 2. 93 X 10 -2 1. 000

+ and – of Sig Dig 1. Identify Estimated Digits. 2. Round all measurements to the left most Estimated Digit.

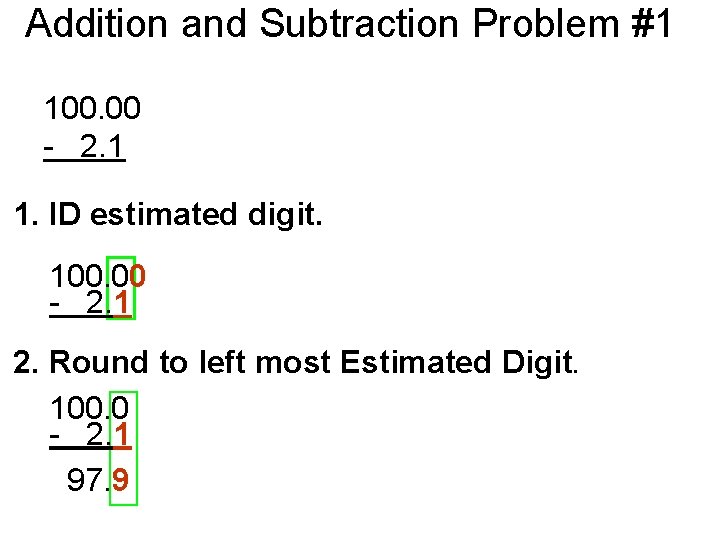
Addition and Subtraction Problem #1 100. 00 - 2. 1 1. ID estimated digit. 100. 00 - 2. 1 2. Round to left most Estimated Digit. 100. 0 - 2. 1 97. 9

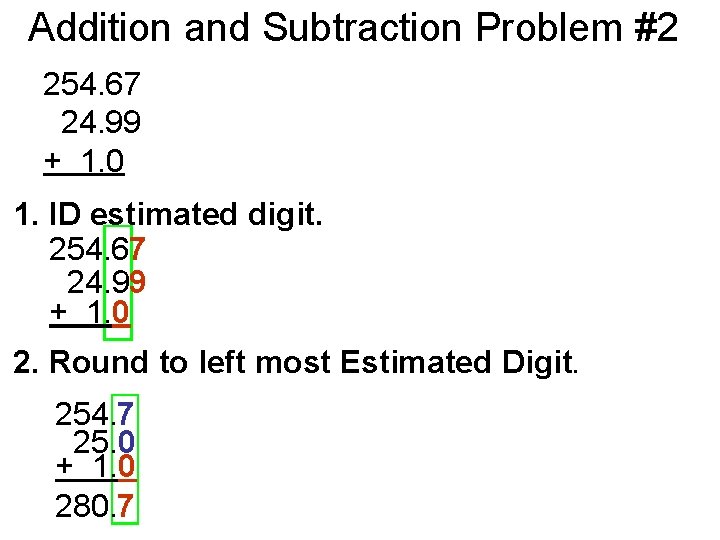
Addition and Subtraction Problem #2 254. 67 24. 99 + 1. 0 1. ID estimated digit. 254. 67 24. 99 + 1. 0 2. Round to left most Estimated Digit. 254. 7 25. 0 + 1. 0 280. 7

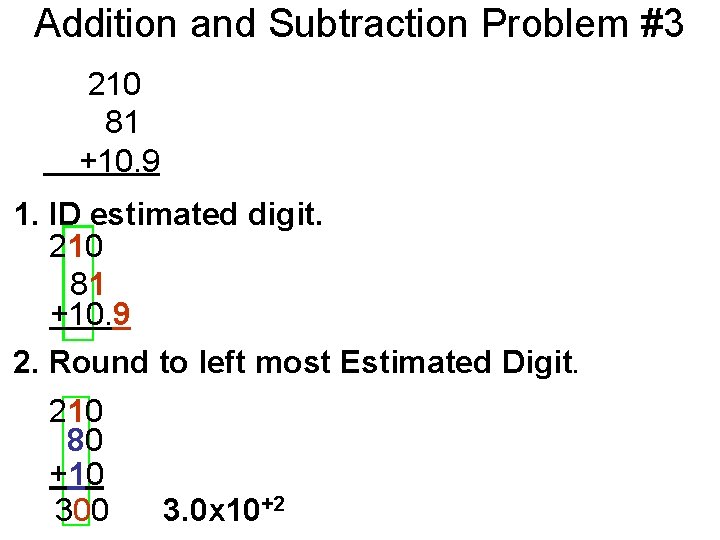
Addition and Subtraction Problem #3 210 81 +10. 9 1. ID estimated digit. 210 81 +10. 9 2. Round to left most Estimated Digit. 210 80 +10 300 3. 0 x 10+2

Addition and Subtraction Problem #4 220 - 81 1. ID estimated digit. 220 - 81 2. Round to left most Estimated Digit. 220 - 80 140

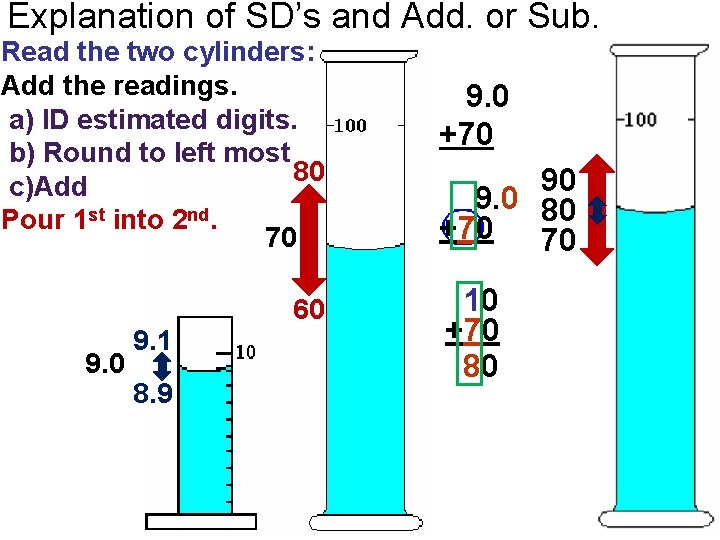
Explanation of SD’s and Add. or Sub. Read the two cylinders: Add the readings. a) ID estimated digits. b) Round to left most 80 c)Add Pour 1 st into 2 nd. 70 9. 1 8. 9 60 9. 0 +70 90 9. 0 80 +70 70 10 +70 80

Notes Three Unit Three • • • Return Quiz Reading Scales For Corrections Review Addition and Subtraction of Significant Digits Quiz Addition and Subtraction of Significant Digits Explain Multiplication and Division of Significant Digits Computer Assignment Multiplication and Division of Significant Digits Pages 56 -64

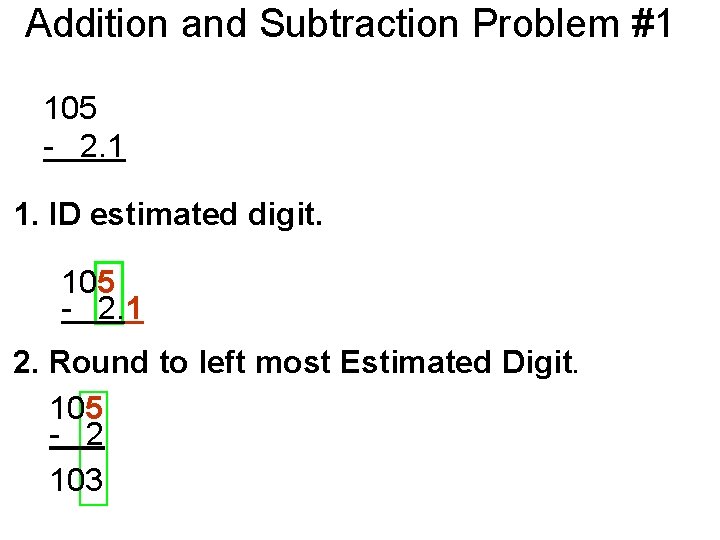
Addition and Subtraction Problem #1 105 - 2. 1 1. ID estimated digit. 105 - 2. 1 2. Round to left most Estimated Digit. 105 - 2 103

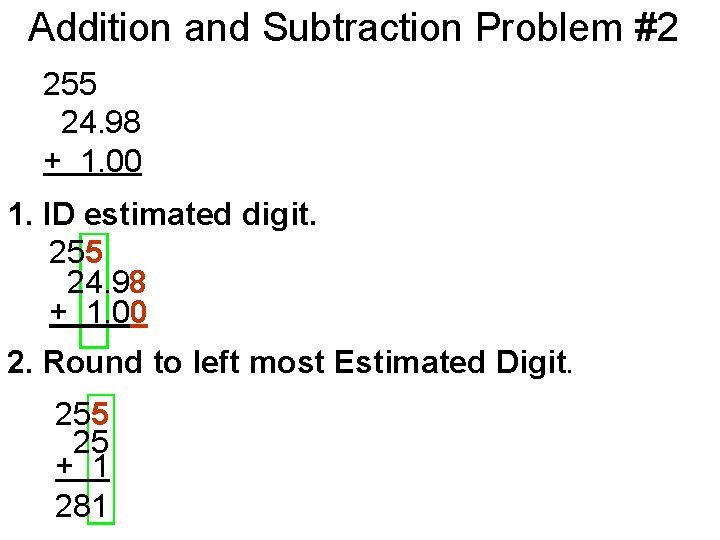
Addition and Subtraction Problem #2 255 24. 98 + 1. 00 1. ID estimated digit. 255 24. 98 + 1. 00 2. Round to left most Estimated Digit. 255 25 + 1 281

Seven Rows Eight Rows

X and ÷ of Sig Dig 1. Identify the number significant digits in each value. 2. Do the indicated math operation. 3. Round to the least number of significant digits.

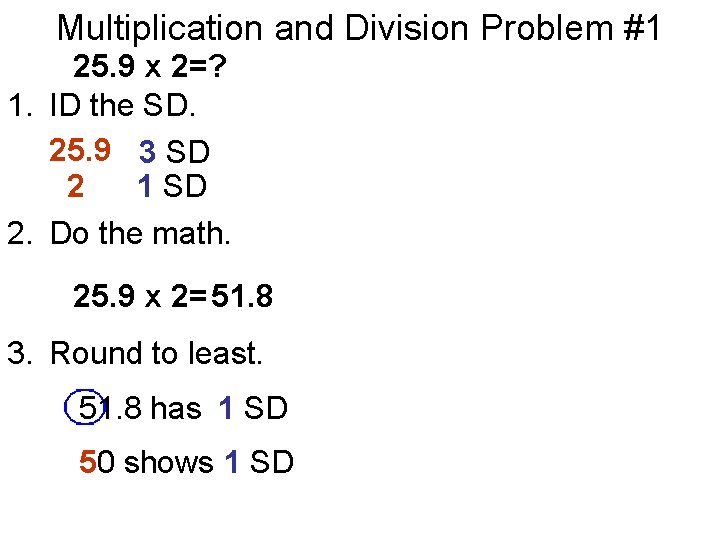
Multiplication and Division Problem #1 25. 9 x 2=? 1. ID the SD. 25. 9 3 SD 2 1 SD 2. Do the math. 25. 9 x 2= 51. 8 3. Round to least. 51. 8 has 1 SD 50 shows 1 SD

Multiplication and Division Problem #2 0. 010 x 325=? 1. ID the SD. 0. 010 2 SD 325 3 SD 2. Do the math. 0. 010 x 325= 3. 25 3. Round to least #. 3. 25 has 2 SD 3. 3 shows 2 SD

Multiplication and Division Problem #3 1200. / 2. 0=? 1. ID the SD. 1200. 4 SD 2. 0 2 SD 2. Do the math. 1200. / 2. 0= 600 3. Round to least #. 600 has 2 SD 6. 0 x 10+2 shows 2 SD

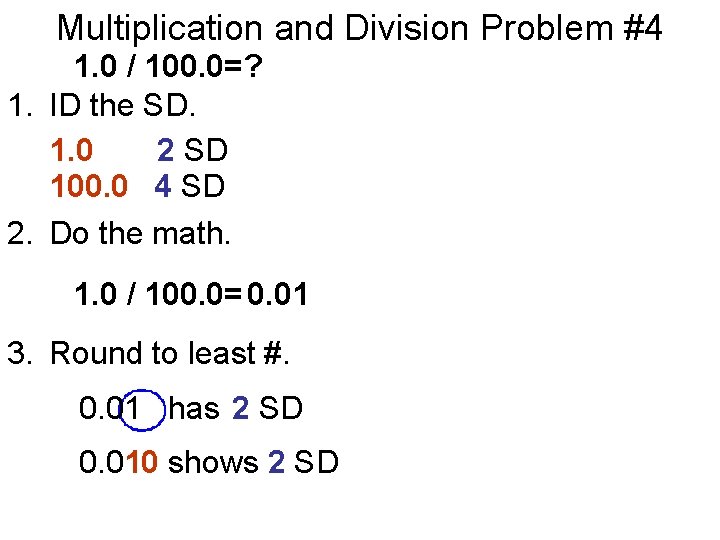
Multiplication and Division Problem #4 1. 0 / 100. 0=? 1. ID the SD. 1. 0 2 SD 100. 0 4 SD 2. Do the math. 1. 0 / 100. 0= 0. 01 3. Round to least #. 0. 01 has 2 SD 0. 010 shows 2 SD

Final Quiz • • Review Lab B results Review For Quiz Cross Word

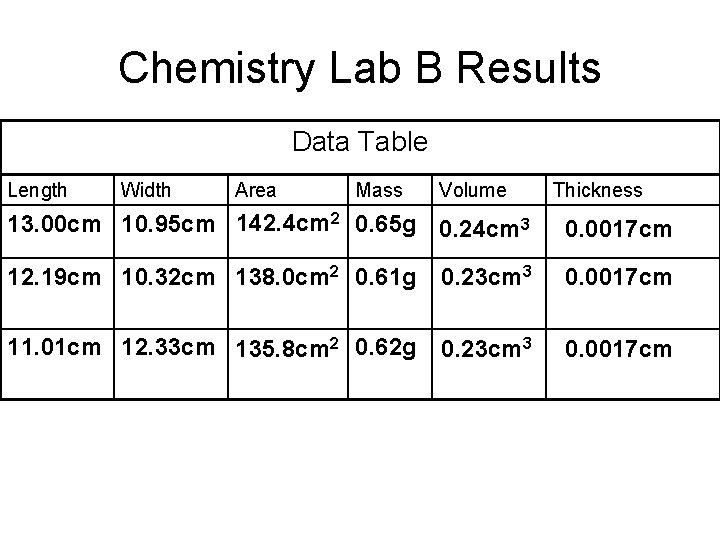
Chemistry Lab B Results Data Table Length Width Area Mass Volume Thickness 13. 00 cm 10. 95 cm 142. 4 cm 2 0. 65 g 0. 24 cm 3 0. 0017 cm 12. 19 cm 10. 32 cm 138. 0 cm 2 0. 61 g 0. 23 cm 3 0. 0017 cm 11. 01 cm 12. 33 cm 135. 8 cm 2 0. 62 g 0. 23 cm 3 0. 0017 cm

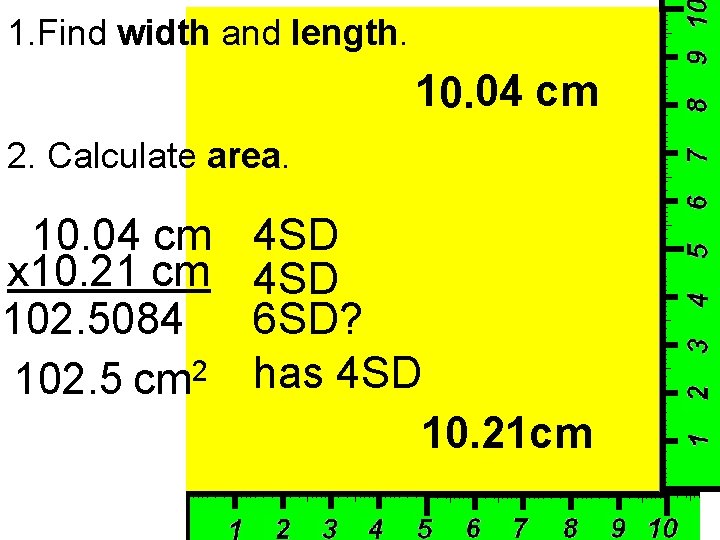
Calculating Thickness of a Gold Sheet 1. 2. 3. 4. 5. Measure the length and width. Calculate the area. Mass the sheet. Divide mass by density to find volume. Divide volume by area to find thickness.

1. Find width and length. 10. 04 cm 2. Calculate area. 10. 04 cm x 10. 21 cm 102. 5084 102. 5 cm 2 4 SD 6 SD? has 4 SD 10. 21 cm

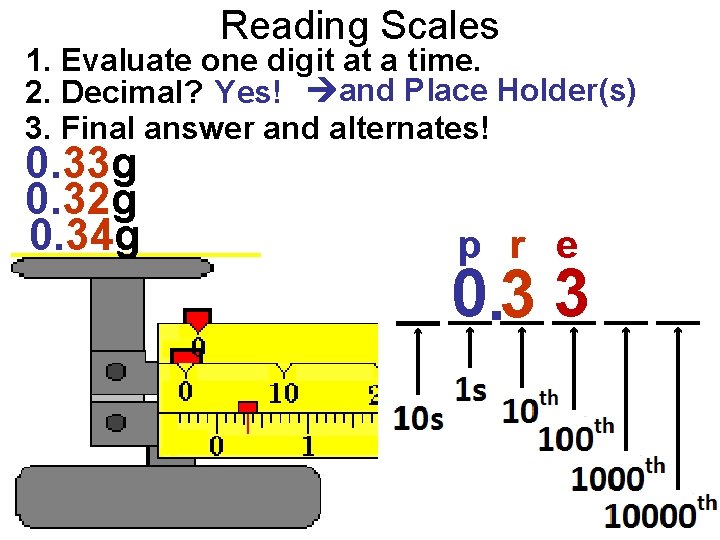
Reading Scales 1. Evaluate one digit at a time. 2. Decimal? Yes! and Place Holder(s) 3. Final answer and alternates! 0. 33 g 0. 32 g 0. 34 g p r e 0. 3 3

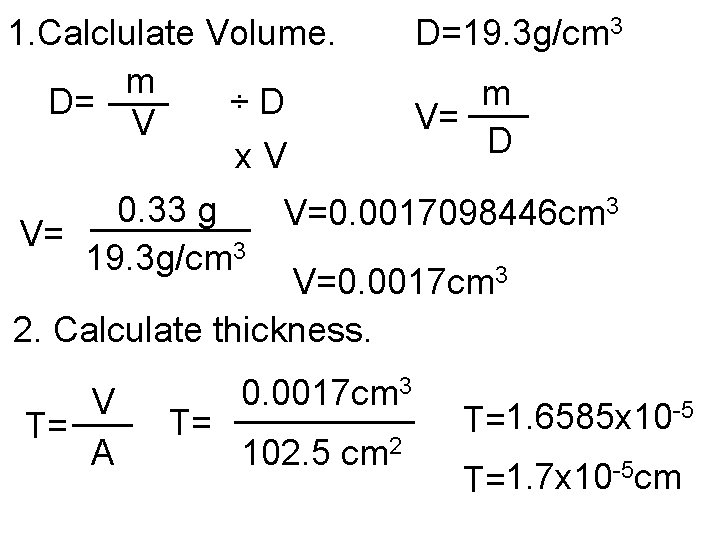
1. Calclulate Volume. D=19. 3 g/cm 3 m ___ D= ÷D V= V D x. V 0. 33 g ____ V=0. 0017098446 cm 3 V= 19. 3 g/cm 3 V=0. 0017 cm 3 2. Calculate thickness. 3 0. 0017 cm V _____ ___ T= T= A 102. 5 cm 2 -5 1. 6585 x 10 T= -5 cm 1. 7 x 10 T=

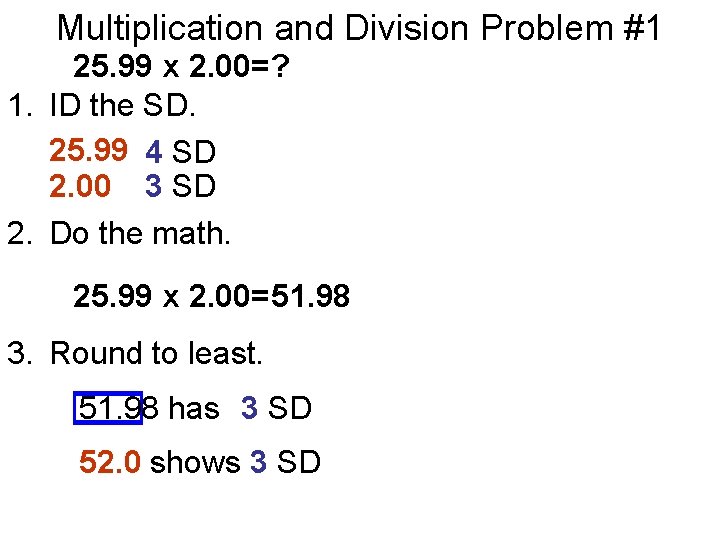
Multiplication and Division Problem #1 25. 99 x 2. 00=? 1. ID the SD. 25. 99 4 SD 2. 00 3 SD 2. Do the math. 25. 99 x 2. 00= 51. 98 3. Round to least. 51. 98 has 3 SD 52. 0 shows 3 SD

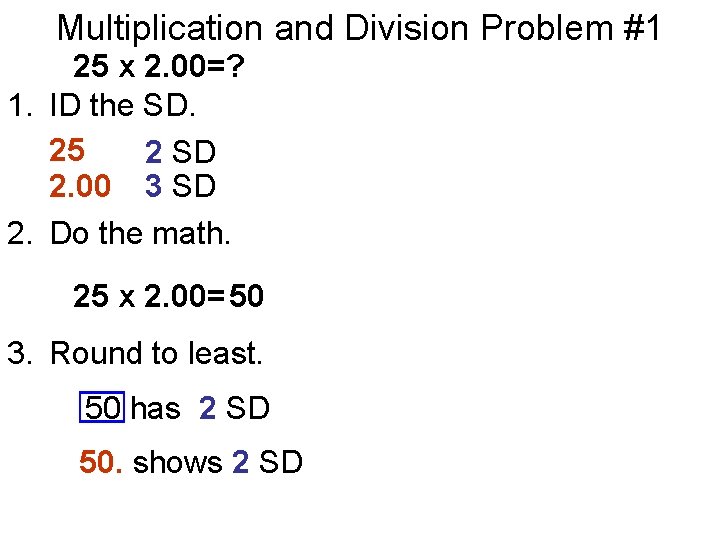
Multiplication and Division Problem #1 25 x 2. 00=? 1. ID the SD. 25 2 SD 2. 00 3 SD 2. Do the math. 25 x 2. 00= 50 3. Round to least. 50 has 2 SD 50. shows 2 SD

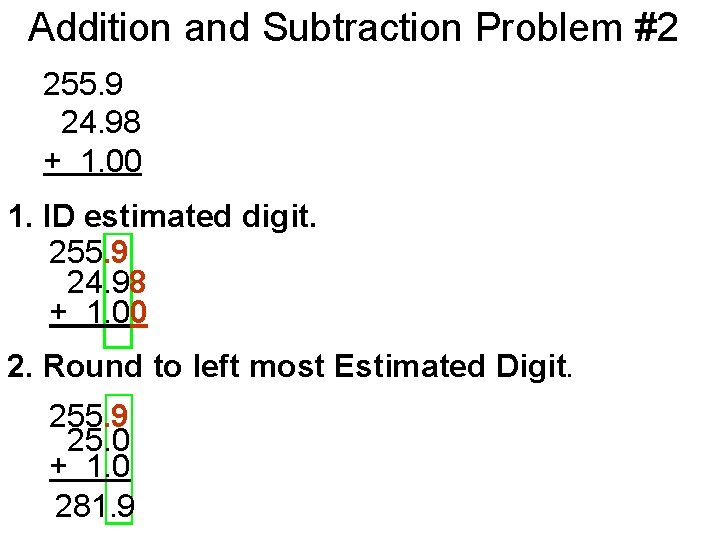
Addition and Subtraction Problem #2 255. 9 24. 98 + 1. 00 1. ID estimated digit. 255. 9 24. 98 + 1. 00 2. Round to left most Estimated Digit. 255. 9 25. 0 + 1. 0 281. 9

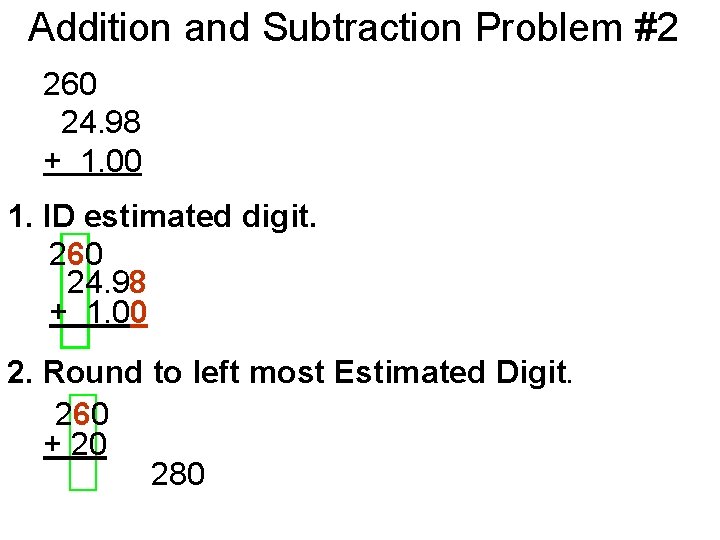
Addition and Subtraction Problem #2 260 24. 98 + 1. 00 1. ID estimated digit. 260 24. 98 + 1. 00 2. Round to left most Estimated Digit. 260 + 20 280


Chemistry Reading Assignment Two 1. What two properties characterize matter? Mass and Volume 2. What SI units are used to express the density of liquids? Grams/Cubic Centimeters(=m. L) 3. How does mass differ from weight? Mass is matter. Weight is the result of gravity acting on mass. 4. Classify the following as either physical or chemical property of matter: area P color P temperature P mass P flammability C odor P oxygen changing to ozone C 5. How does an atom differ from an element? Atoms that have the same proton number are all the same element. 6. How is an atom related to a molecule? Atoms make up molecules: Atoms of hydrogen and oxygen combine to form water. 7. Define each: Mixture- Two or more substances stirred together. Homogeneous- Substances mix so well that they appear as one. Heterogeneous- Substances will not mix. One can see the parts.

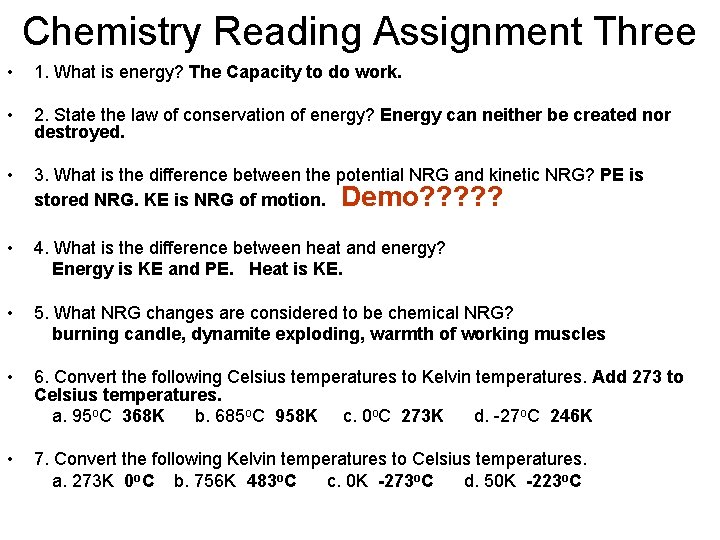
Chemistry Reading Assignment Three • 1. What is energy? The Capacity to do work. • 2. State the law of conservation of energy? Energy can neither be created nor destroyed. • 3. What is the difference between the potential NRG and kinetic NRG? PE is stored NRG. KE is NRG of motion. Demo? ? ? • 4. What is the difference between heat and energy? Energy is KE and PE. Heat is KE. • 5. What NRG changes are considered to be chemical NRG? burning candle, dynamite exploding, warmth of working muscles • 6. Convert the following Celsius temperatures to Kelvin temperatures. Add 273 to Celsius temperatures. a. 95 o. C 368 K b. 685 o. C 958 K c. 0 o. C 273 K d. -27 o. C 246 K • 7. Convert the following Kelvin temperatures to Celsius temperatures. a. 273 K 0 o. C b. 756 K 483 o. C c. 0 K -273 o. C d. 50 K -223 o. C

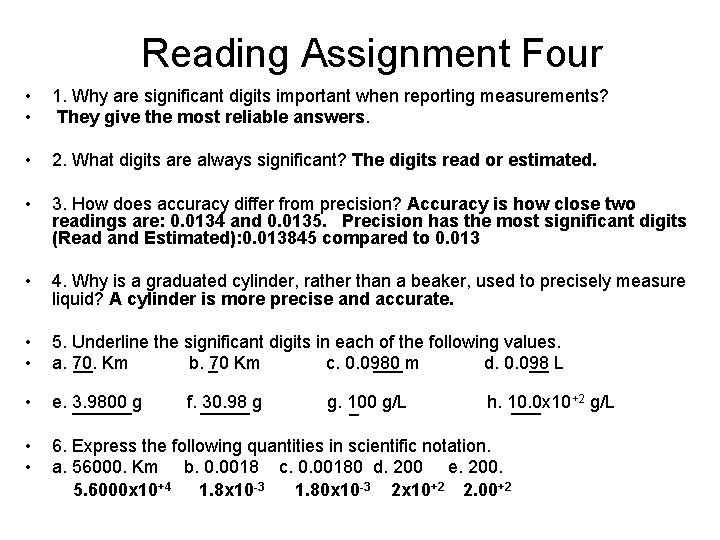
Reading Assignment Four • • 1. Why are significant digits important when reporting measurements? They give the most reliable answers. • 2. What digits are always significant? The digits read or estimated. • 3. How does accuracy differ from precision? Accuracy is how close two readings are: 0. 0134 and 0. 0135. Precision has the most significant digits (Read and Estimated): 0. 013845 compared to 0. 013 • 4. Why is a graduated cylinder, rather than a beaker, used to precisely measure liquid? A cylinder is more precise and accurate. • • 5. Underline the significant digits in each of the following values. __ Km ___ m __ L a. 70. b. _ 70 Km c. 0. 0980 d. 0. 098 • e. ______ 3. 9800 g • • 6. Express the following quantities in scientific notation. a. 56000. Km b. 0. 0018 c. 0. 00180 d. 200 e. 200. 5. 6000 x 10+4 1. 8 x 10 -3 1. 80 x 10 -3 2 x 10+2 2. 00+2 f. _____ 30. 98 g g. 100 _ g/L +2 g/L h. 10. 0 x 10 ___

Reading Scales • In order to produce reliable data…density, volume, mass…. we need to be able to read the scales on measuring devices. 1. Place Water in the Erlenmeyer flask…Record the reading. 2. Pour the water into the smaller cylinder…Record the reading. 3. Pour the water to the larger cylinder…Record the reading. 4. Measure the mass of the piece of metal using the balance Record the reading. Pages 56 -64

SIGNIFICANT DIGIT RULES • 1. All non zero digits are significant. • 2. Any digit, including zero, read or estimated from a scale is significant. • 3. Leading zeroes in numbers are never significant: the three first zeroes in 0. 0010 are leading zeroes; therefore, they are not significant. They are, also, never read or estimated form a scale. Leading zeroes show place value. • 4. Trailing zeroes are significant if a decimal is present. The zeroes in 400. 0 and 400. are identified as significant by the decimal. Leading zeroes cannot be made significant by a decimal. • 5. Only significant digits can be expressed in scientific notation

Reading Scales 1. Evaluate one digit at a time. 2. Decimal? YES! No Place Holder(s)! 3. Final answer and alternates! 10. 0 9. 9 10. 1 r r e 1 0. 0