Chapter 2 Section 2 Measuring Matter Weight mass


























- Slides: 48

Chapter 2, Section 2 “Measuring Matter” Weight, mass, volume & density

Section 2 Measuring Matter OBJECTIVES: l Distinguish between the mass and weight of an object.

The International System of Units OBJECTIVES: l. List SI units of measurement and common SI prefixes.

Mass vs Weight

Units of Mass § Mass is a measure of the quantity of matter present § Weight is a force that measures the pull by gravity- it changes with location § Mass is constant, regardless of location

Weight is the Pull of Gravity Weight is a measure of the pull of gravity. Weight is measured with a spring that is compressed or stretched.

All Matter has Gravity The elephant has more matter so it has more mass, so it has more gravity. Even dust is matter so it pulls other dust together by gravity.

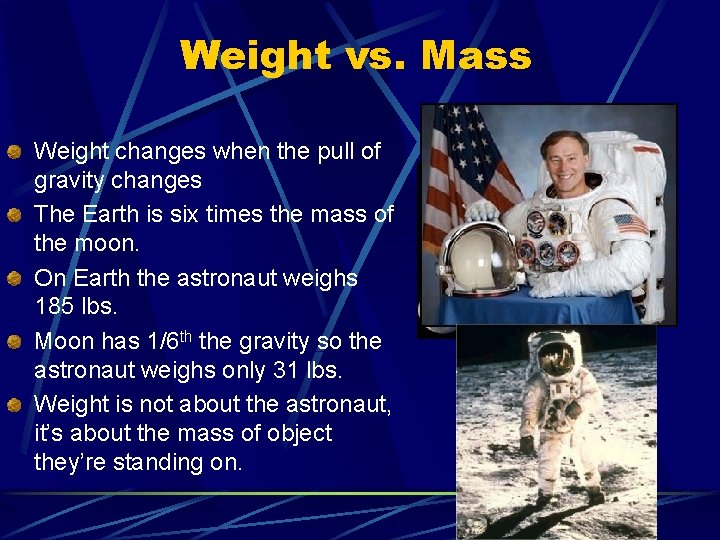
Weight vs. Mass Weight changes when the pull of gravity changes The Earth is six times the mass of the moon. On Earth the astronaut weighs 185 lbs. Moon has 1/6 th the gravity so the astronaut weighs only 31 lbs. Weight is not about the astronaut, it’s about the mass of object they’re standing on.

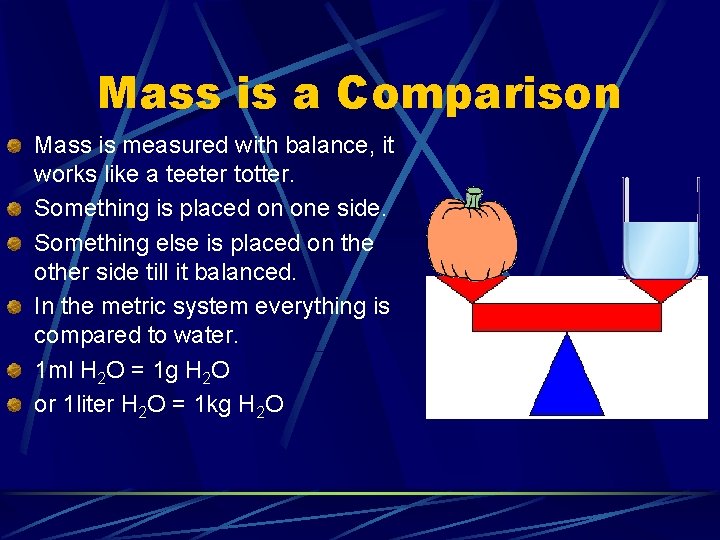
Mass is a Comparison Mass is measured with balance, it works like a teeter totter. Something is placed on one side. Something else is placed on the other side till it balanced. In the metric system everything is compared to water. 1 ml H 2 O = 1 g H 2 O or 1 liter H 2 O = 1 kg H 2 O

Mass Does Not Change While on the Earth I will place an object on one side of a balance and an equal amount of water on the other side. Now I will travel to the moon. Once on the moon I will repeat the experiment. Although the moon pulls 1/6 th as hard on the object, it also pulls 1/6 th as hard on the water, so it still balances. For this reason scientists prefer to measure matter by it’s mass not weight.

Working with Mass § The SI unit of mass is the kilogram (kg), even though a more convenient everyday unit is the gram § Measuring instrument is the balance scale

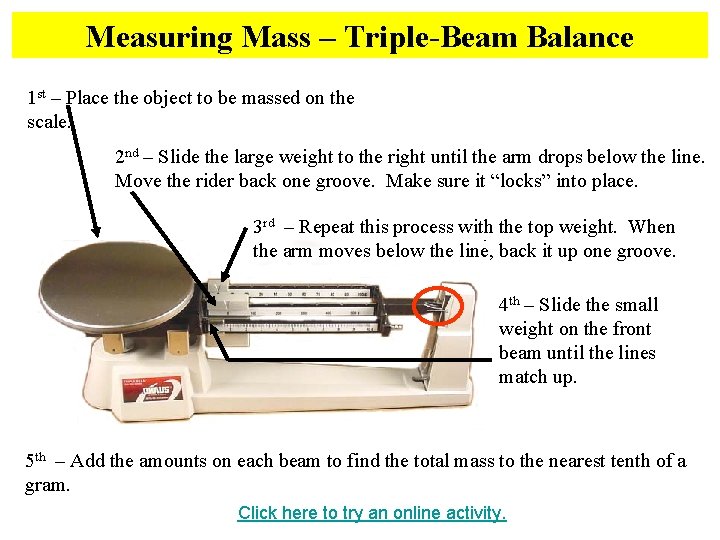
Measuring Mass – Triple-Beam Balance 1 st – Place the object to be massed on the scale. 2 nd – Slide the large weight to the right until the arm drops below the line. Move the rider back one groove. Make sure it “locks” into place. 3 rd – Repeat this process with the top weight. When the arm moves below the line, back it up one groove. 4 th – Slide the small weight on the front beam until the lines match up. 5 th – Add the amounts on each beam to find the total mass to the nearest tenth of a gram. Click here to try an online activity.

Measuring Mass We will be using triple-beam balances to find the mass of various objects. The objects are placed on the scale and then you move the weights on the beams until you get the lines on the right-side of the scale to match up. Once you have balanced the scale, you add up the amounts on each beam to find the total mass. What would be the mass of the object measured in the picture? _______ + _______ = ____ g Top Image: http: //www. southwestscales. com/Ohaus_Triple_Beam_750 -SO. jpg Bottom Image: http: //www. regentsprep. org/Regents/biology/units/laboratory/graphics/triplebeambalance. jpg

Measuring The numbers are only half of a measurement l Measurement = number + unit It has a mass of 10 10 what? ? Numbers without units are meaningless. You will lose credit is you do not include your units!

The International System of Units OBJECTIVES: l. List SI units of measurement and common SI prefixes.

Section 2 Measuring Matter OBJECTIVES: l Identify the units of volume & density l Explain how density is determined

International System of Units § Measurements depend upon units that serve as reference standards § The standards of measurement used in science are those of the Metric System

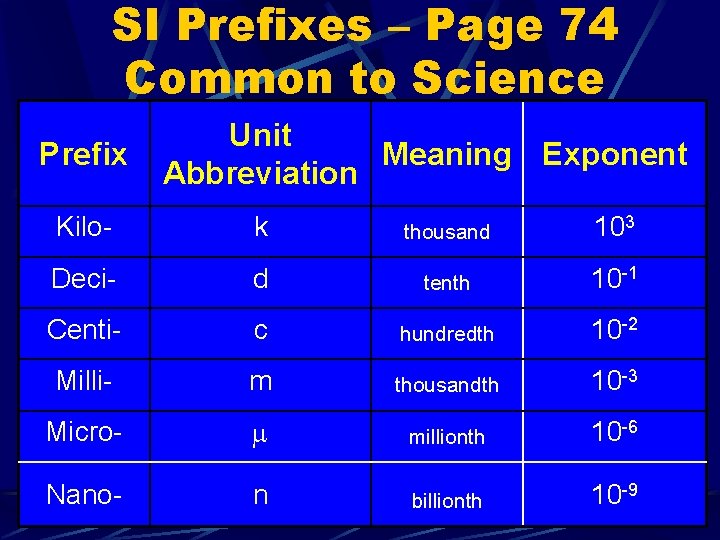
The Metric System Easier to use because it is a decimal system Every conversion is by some power of 10. A metric unit has two parts A prefix and a base unit. prefix tells you how many times to divide or multiply by 10.

Length § In SI, the basic unit of length is the meter (m) § Length is the distance between two objects – measured with ruler § We make use of prefixes for units larger or smaller

SI Prefixes – Page 74 Common to Science Prefix Unit Meaning Exponent Abbreviation Kilo- k thousand 103 Deci- d tenth 10 -1 Centi- c hundredth 10 -2 Milli- m thousandth 10 -3 Micro- millionth 10 -6 Nano- n billionth 10 -9

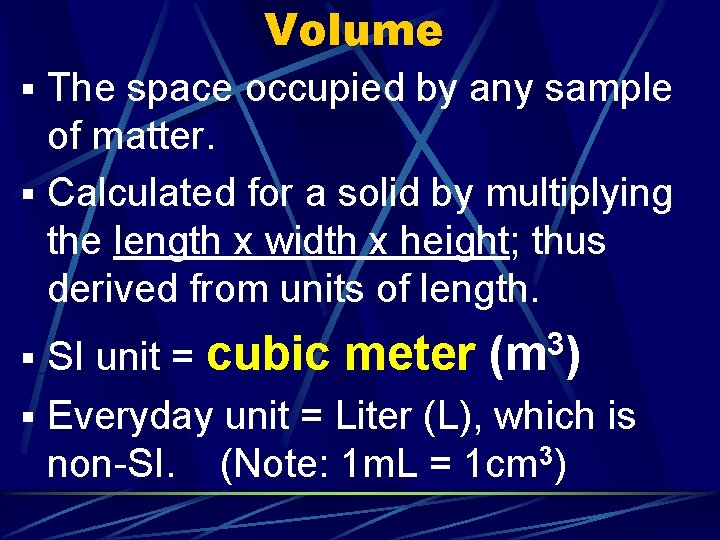
Volume § The space occupied by any sample of matter. § Calculated for a solid by multiplying the length x width x height; thus derived from units of length. § SI unit = cubic meter 3 (m ) § Everyday unit = Liter (L), which is non-SI. (Note: 1 m. L = 1 cm 3)

Recap & Review § Volume is: § The space occupied by any sample of matter. § Mass is: § The amount of matter in an object § Weight is: § The force (or pull) of gravity on an object based on its mass § Which changes when your on the moon, your mass or weight? § Your weight § What is the SI unit for: Mass, Volume and Length § Mass = kg (kilogram) § Volume = m 3 (cubic meter), we will use liters and milliliters in this class § Length = m (meter) § What is bigger a millimeter or a kilometer? By how much? § A kilometer (1000 meters) is bigger § A millimeter is 1/1000 of a meter so a kilometer is 1 million times bigger

Section 2 Measuring Volume & Density OBJECTIVES: Define what density is l Identify the units of volume & density l Explain how density is determined l

Measuring Volume & Density § Volume is: § The space occupied by any sample of matter. § Mass is: § The amount of matter in an object § Density is ? ? ?

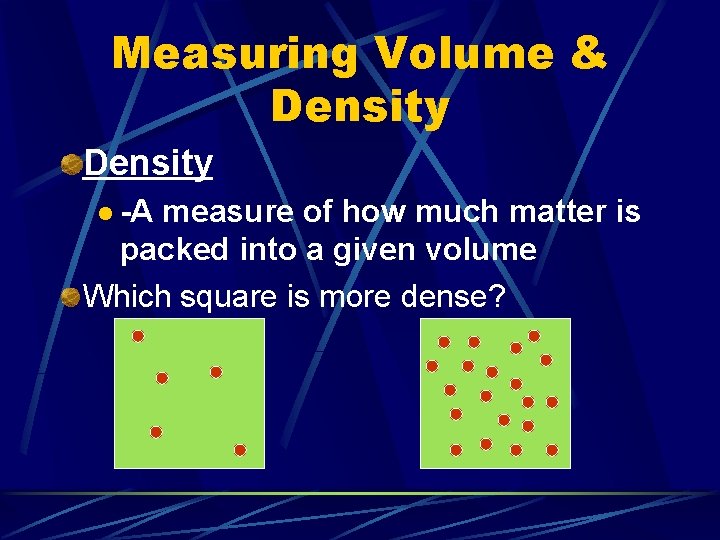
Measuring Volume & Density l -A measure of how much matter is packed into a given volume Which square is more dense?

Which one is more dense? Now which one is more dense?

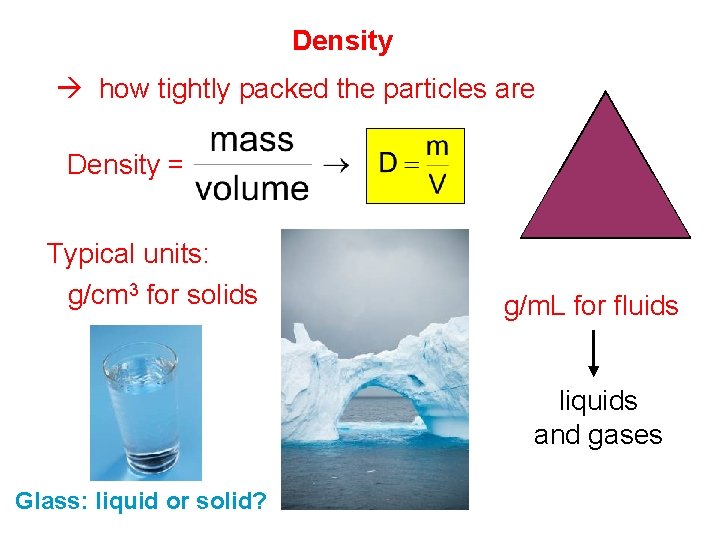
Density how tightly packed the particles are m Density = D Typical units: g/cm 3 for solids V g/m. L for fluids liquids and gases Glass: liquid or solid?

Density § Which is heavier- a pound of lead or a pound of feathers? § Most people will answer lead, but the weight is exactly the same § They are normally thinking about equal volumes of the two § The relationship here between mass and volume is called Density

Density § The formula for density is: mass Density = volume g/m. L, or possibly g/cm 3, (or g/L for gas) • Common units are: • Density is a physical property, and does not depend upon sample size

Measuring Volume We will be using graduated cylinders to find the volume of liquids and other objects. Read the measurement based on the bottom of the meniscus or curve. When using a real cylinder, make sure you are eye-level with the level of the water. What is the volume of water in the cylinder? _____m. L What causes the meniscus? A concave meniscus occurs when the molecules of the liquid attract those of the container. The glass attracts the water on the sides. Top Image: http: //www. tea. state. tx. us/student. assessment/resources/online/2006/grade 8/science/images/20 graphicaa. gif Bottom Image: http: //morrisonlabs. com/meniscus. htm

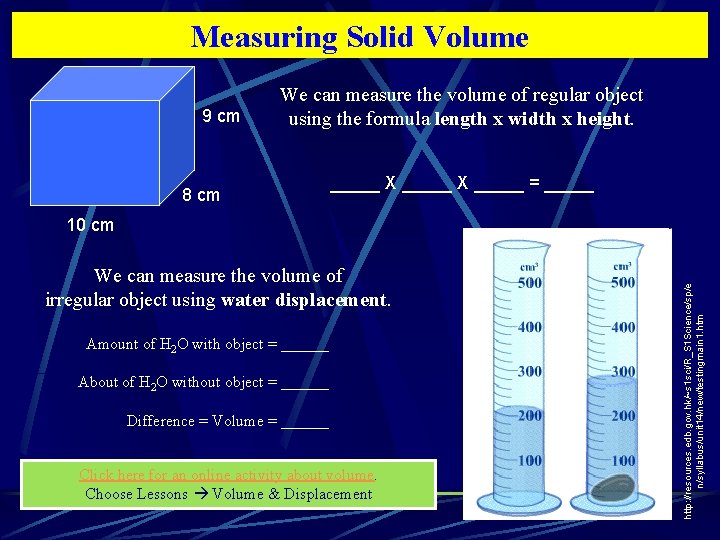
Measuring Solid Volume 9 cm We can measure the volume of regular object using the formula length x width x height. 8 cm _____ X _____ = _____ We can measure the volume of irregular object using water displacement. Amount of H 2 O with object = ______ About of H 2 O without object = ______ Difference = Volume = ______ Click here for an online activity about volume. Choose Lessons Volume & Displacement http: //resources. edb. gov. hk/~s 1 sci/R_S 1 Science/sp/e n/syllabus/unit 14/new/testingmain 1. htm 10 cm

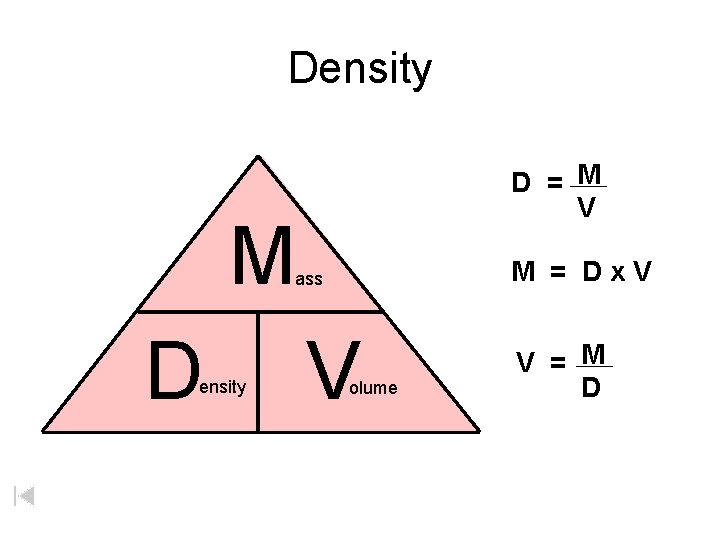
Density D = M V M M = Dx. V ass D ensity V olume V = M D

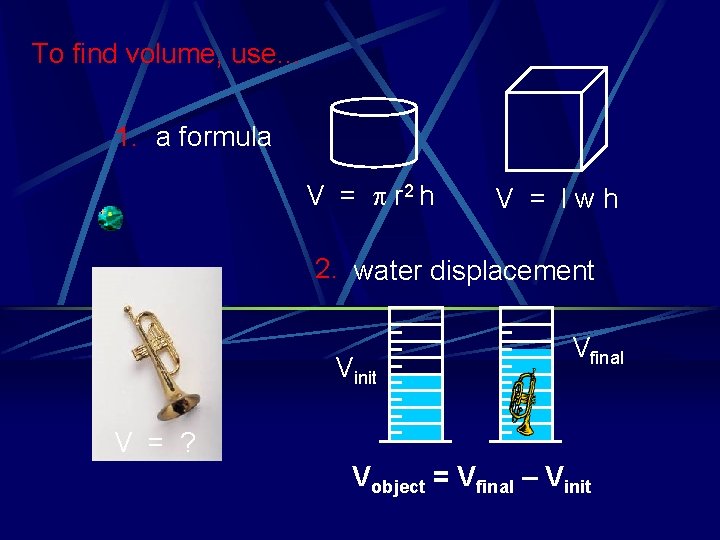
To find volume, use… 1. a formula V = p r 2 h V = lwh 2. water displacement Vinit Vfinal V = ? Vobject = Vfinal – Vinit

** Density of water = 1. 0 g/m. L = 1. 0 g/cm 3 Things that are “less dense” float in things that are “more dense. ” (And things that are “more dense” sink in things that are “less dense. ”) D < 1 g/cm 3 D > 1 g/cm 3 D < 1 g/cm 3 The density of a liquid or solid is nearly constant, no matter what the sample’s temperature. Density of gases is highly dependent on temperature.

So why is an aircraft carrier less dense than water? • It’s mass of steel is spread across a huge volume with lots of empty space in between • Since Density = Mass/Volume • The mass of the steel spread across the huge volume gives a density < 1. 0 g/cm 3 (less than water) D < 1 g/cm 3 *Things that are “less dense” float in things that are “more dense. ”

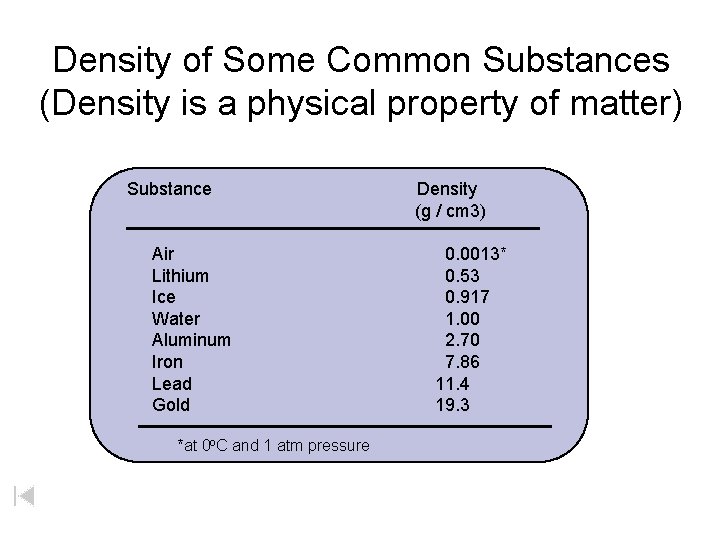
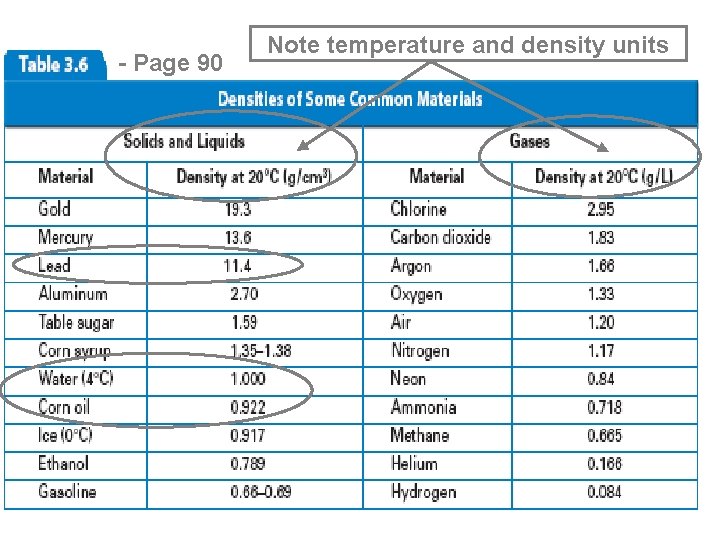
Density of Some Common Substances (Density is a physical property of matter) Substance Air Lithium Ice Water Aluminum Iron Lead Gold *at 0 o. C and 1 atm pressure Density (g / cm 3) 0. 0013* 0. 53 0. 917 1. 00 2. 70 7. 86 11. 4 19. 3

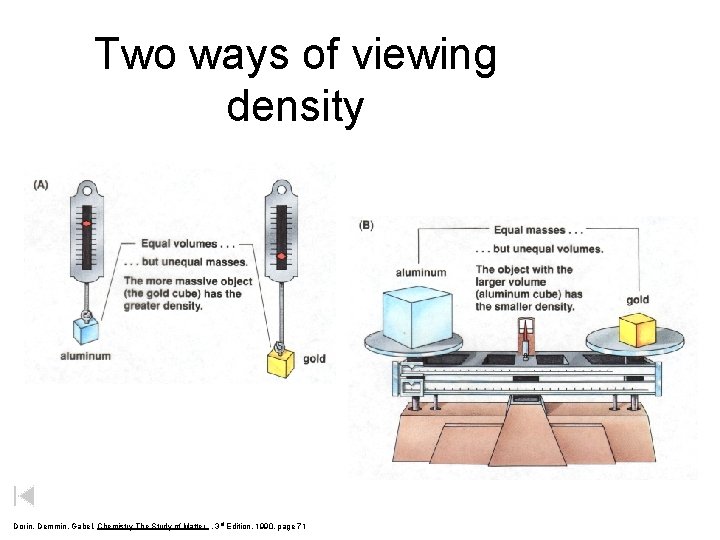
Two ways of viewing density Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3 rd Edition, 1990, page 71

Density and Temperature § What happens to the density as the temperature of an object increases? § Mass remains the same § Most substances increase in volume as temperature increases § Thus, density generally decreases as the temperature increases

Density and Water § Water is an important exception to the previous statement. § Over certain temperatures, the volume of water increases as the temperature decreases (Do you want your water pipes to freeze in the winter? ) § Does ice float in liquid water? § Why?

- Page 90 Note temperature and density units

Measure Density for yourself • Complete Skills Lab: Making Sense of Density • Complete skills lab data table & questions (Due tomorrow) • Complete Measuring Matter and Density Work Sheet (Due Friday)

Density Review

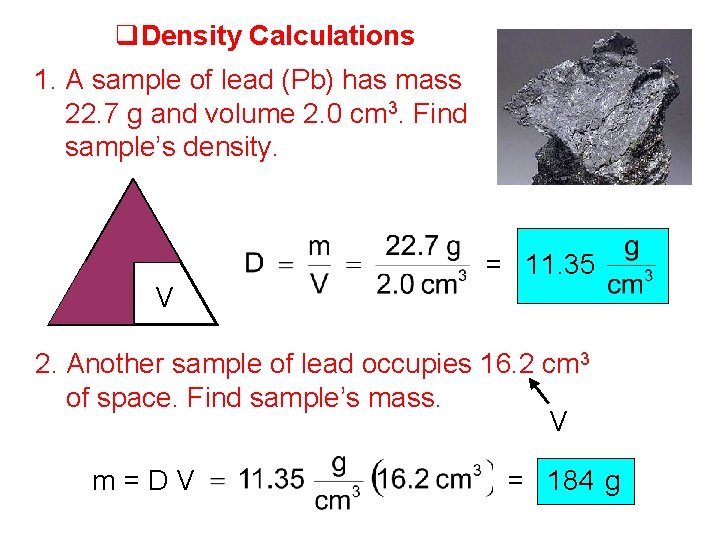
Density Calculations 1. A sample of lead (Pb) has mass 22. 7 g and volume 2. 0 cm 3. Find sample’s density. m D = 11. 35 V 2. Another sample of lead occupies 16. 2 cm 3 of space. Find sample’s mass. V m=DV = 184 g

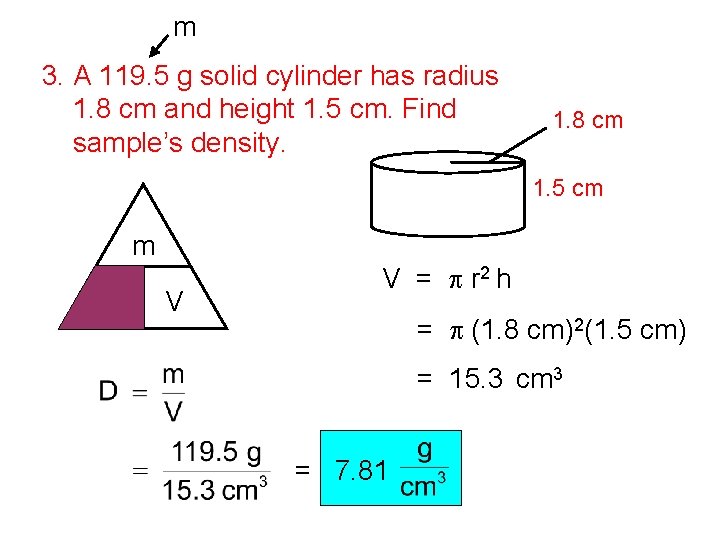
m 3. A 119. 5 g solid cylinder has radius 1. 8 cm and height 1. 5 cm. Find sample’s density. 1. 8 cm 1. 5 cm m D V V = p r 2 h = p (1. 8 cm)2(1. 5 cm) = 15. 3 cm 3 = 7. 81

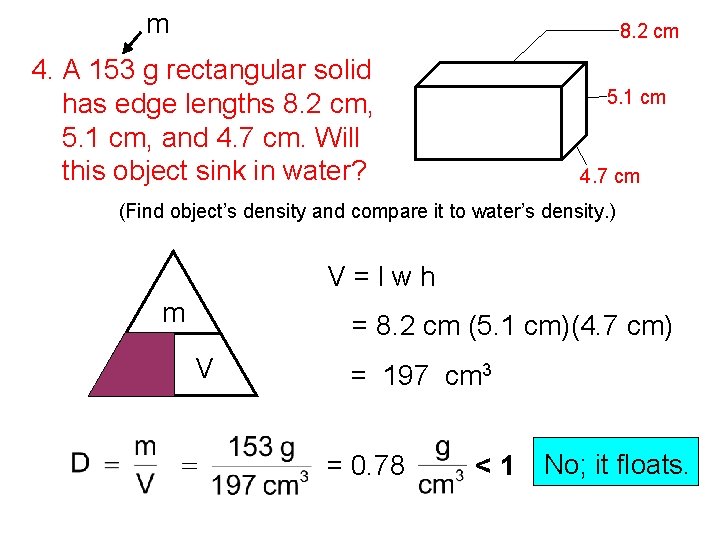
m 8. 2 cm 4. A 153 g rectangular solid has edge lengths 8. 2 cm, 5. 1 cm, and 4. 7 cm. Will this object sink in water? 5. 1 cm 4. 7 cm (Find object’s density and compare it to water’s density. ) V=lwh m D = 8. 2 cm (5. 1 cm)(4. 7 cm) V = 197 cm 3 = 0. 78 <1 No; it floats.

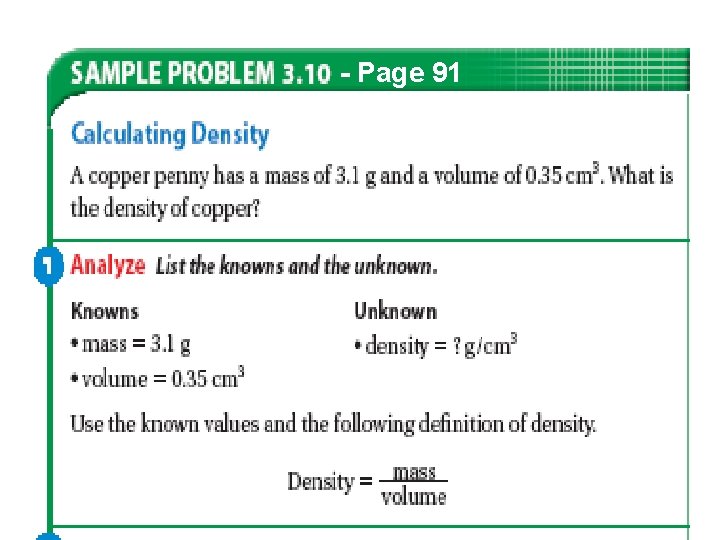
- Page 91

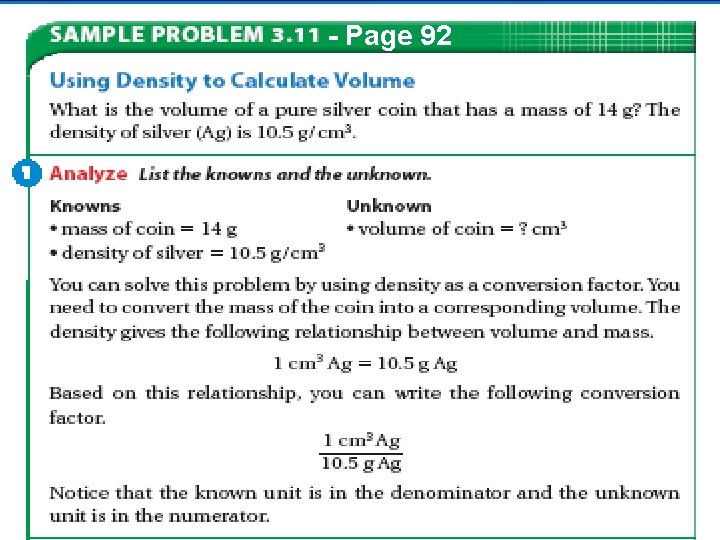
- Page 92
