Phases of Matter Video on States of Matter






- Slides: 19

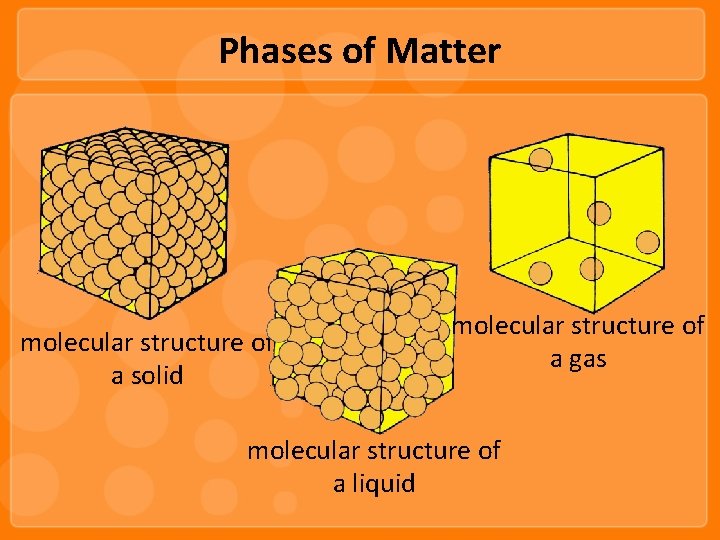
Phases of Matter

Video on States of Matter

Questions 1 • What is the fourth state of matter? • Which of the states of matter has fixed volume? • Which of the states of matter has no structure? • Which is most abundant? • What does the word gas mean? Why is it fitting to call it gas?

Questions 2 • What benefits are derived from understanding the fourth state of matter? • What are the many forms of solid water? • What are the different guises of gaseous water? • How does liquid water become gas? • What term is used to describe gaseous water turning into liquid?

Phases of Matter molecular structure of a solid molecular structure of a gas molecular structure of a liquid

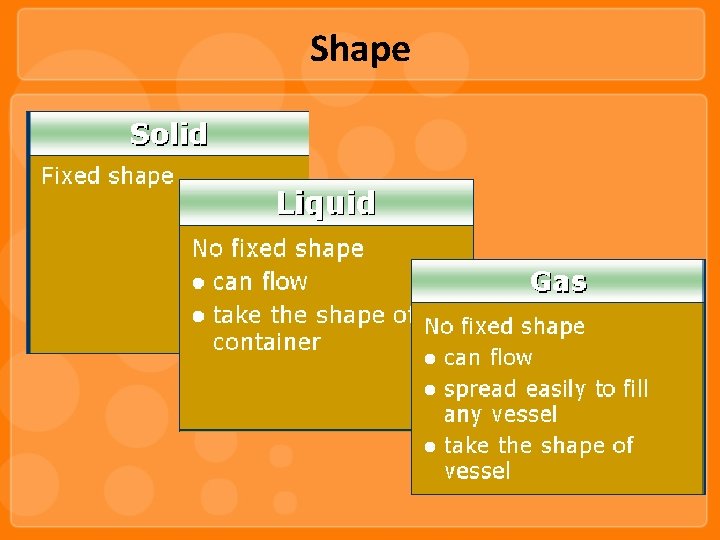
Shape

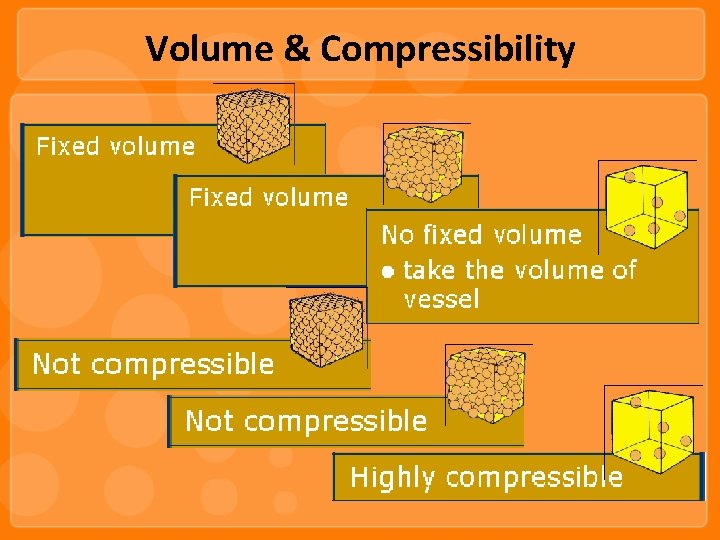
Volume & Compressibility

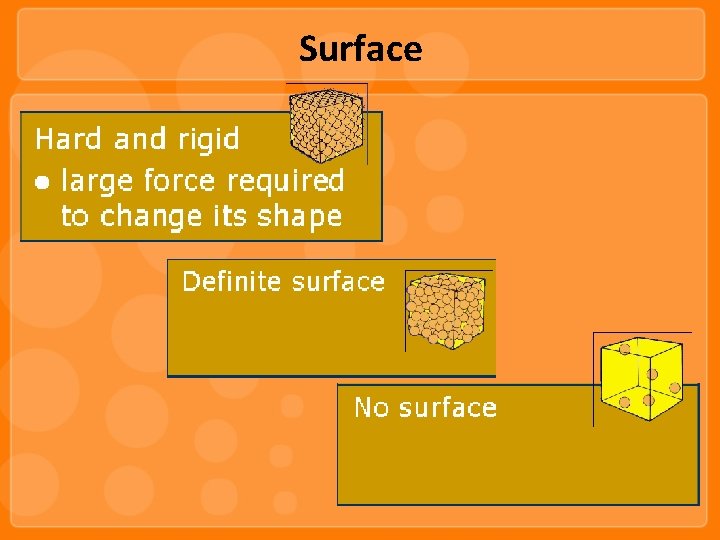
Surface

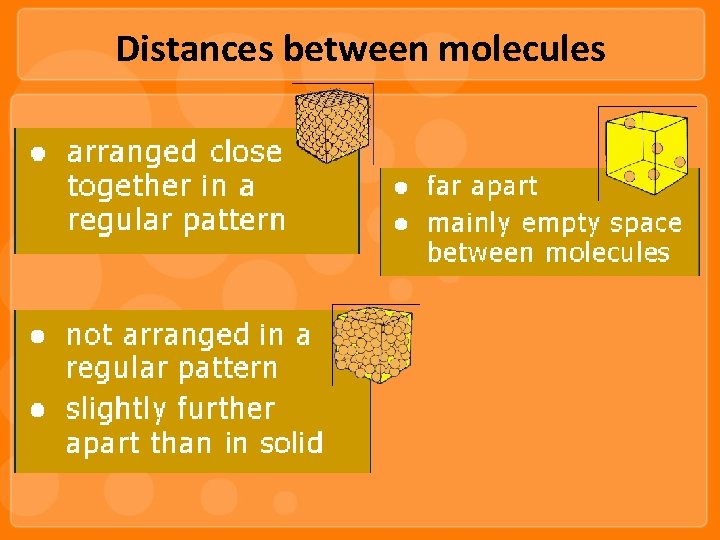
Distances between molecules

Which is most likely oxygen gas? A B C

Which is most likely liquid water? A B C

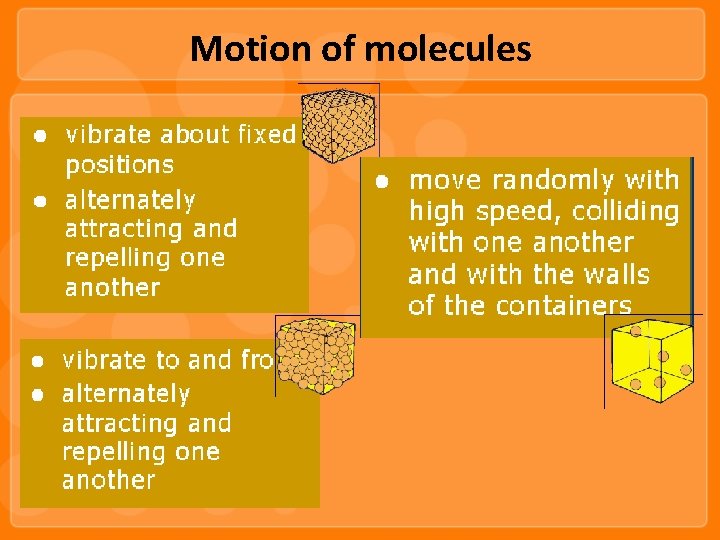
Motion of molecules

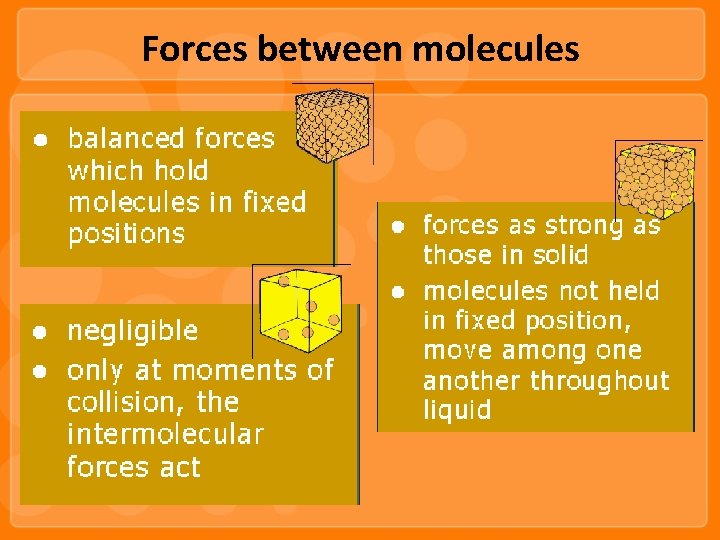
Forces between molecules

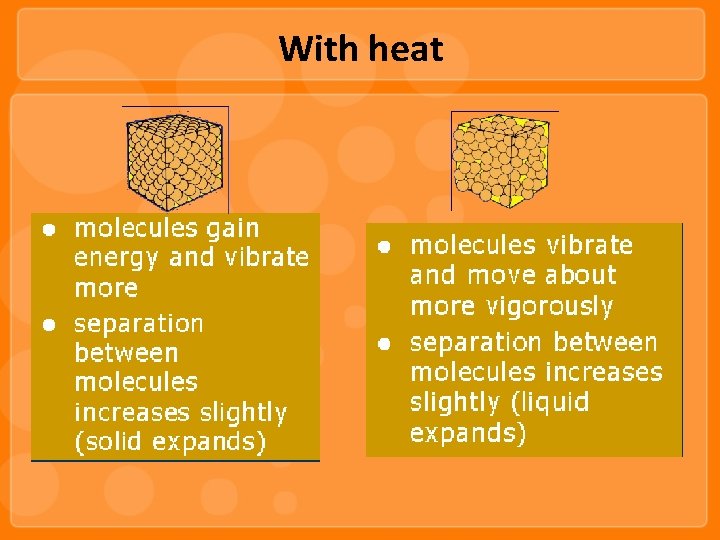
With heat

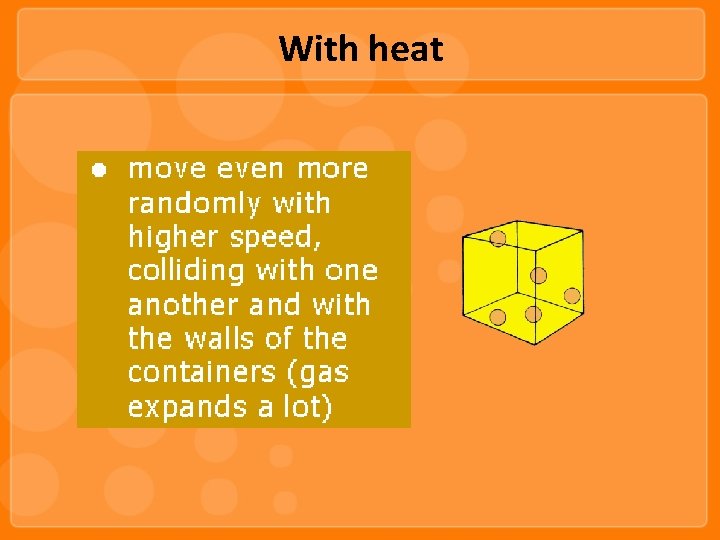
With heat

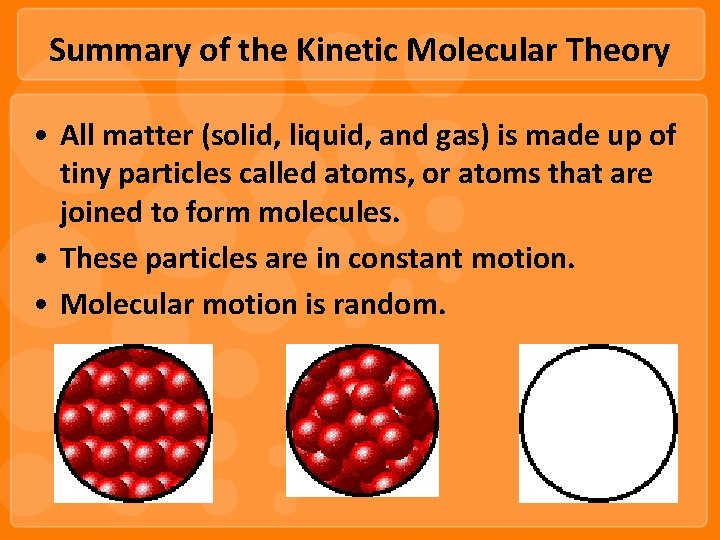
Summary of the Kinetic Molecular Theory • All matter (solid, liquid, and gas) is made up of tiny particles called atoms, or atoms that are joined to form molecules. • These particles are in constant motion. • Molecular motion is random.

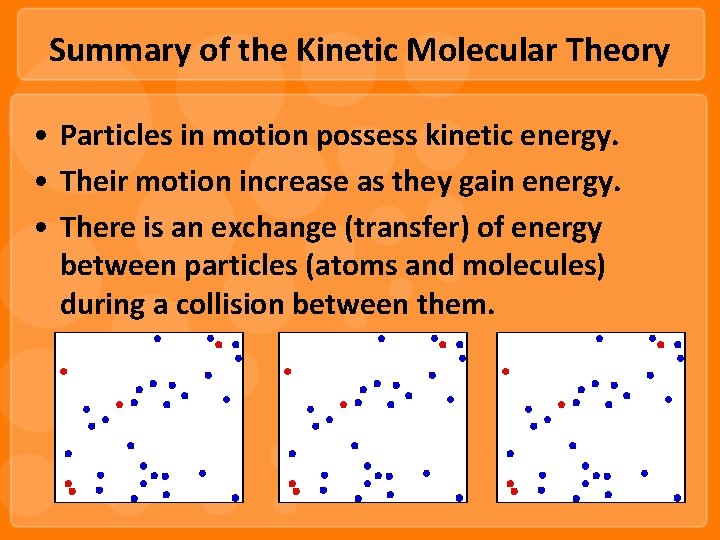
Summary of the Kinetic Molecular Theory • Particles in motion possess kinetic energy. • Their motion increase as they gain energy. • There is an exchange (transfer) of energy between particles (atoms and molecules) during a collision between them.

Summary of the Kinetic Molecular Theory • Particles (molecules) in gases do not exert large forces on each other, unless they are in collision with each other. • Collisions between these particles are perfectly elastic.

Summary of the Kinetic Molecular Theory • The average kinetic energy of the particles depends only on the temperature of the system.
 Video production 101
Video production 101 Phases of matter
Phases of matter 3 phases of matter
3 phases of matter Concept map of matter solid liquid and gas
Concept map of matter solid liquid and gas Four states of matter
Four states of matter Why isn't it a good idea to classify matter by its phases
Why isn't it a good idea to classify matter by its phases 4 phases of matter
4 phases of matter Whats the study of matter and energy
Whats the study of matter and energy Phases changes of matter
Phases changes of matter Yahoo search video
Yahoo search video The frame size of a video refers to the video’s
The frame size of a video refers to the video’s Textd
Textd Yahoo gravity
Yahoo gravity Tyranny
Tyranny Southern vs northern states
Southern vs northern states What were the 11 free states
What were the 11 free states Examples of gas
Examples of gas All states of matter
All states of matter States of matter jeopardy
States of matter jeopardy States of matter: basics
States of matter: basics