Percent Composition and Molecular Vs Empirical Formulas 1
- Slides: 17

Percent Composition and Molecular Vs. Empirical Formulas 1

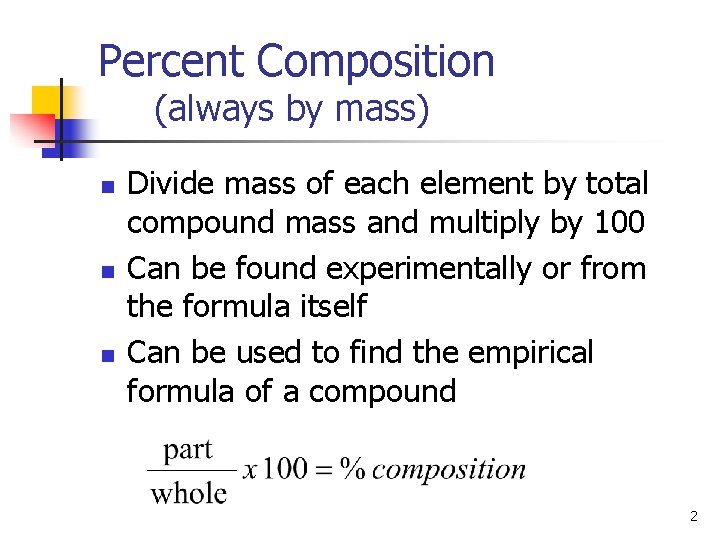
Percent Composition (always by mass) n n n Divide mass of each element by total compound mass and multiply by 100 Can be found experimentally or from the formula itself Can be used to find the empirical formula of a compound 2

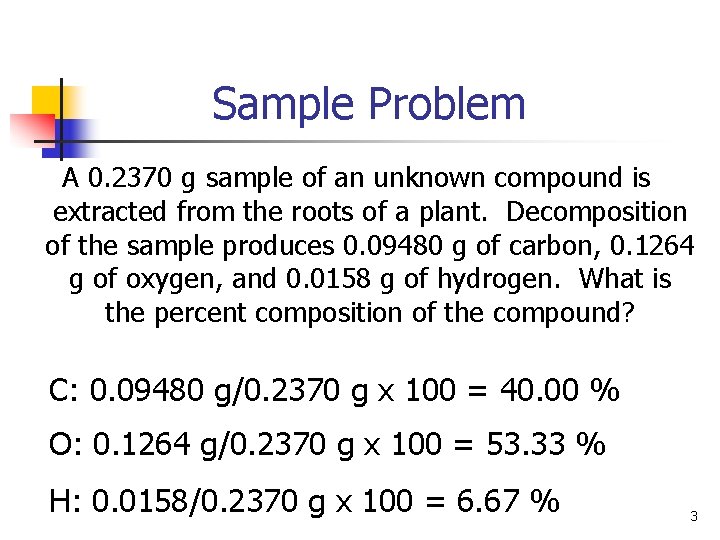
Sample Problem A 0. 2370 g sample of an unknown compound is extracted from the roots of a plant. Decomposition of the sample produces 0. 09480 g of carbon, 0. 1264 g of oxygen, and 0. 0158 g of hydrogen. What is the percent composition of the compound? C: 0. 09480 g/0. 2370 g x 100 = 40. 00 % O: 0. 1264 g/0. 2370 g x 100 = 53. 33 % H: 0. 0158/0. 2370 g x 100 = 6. 67 % 3

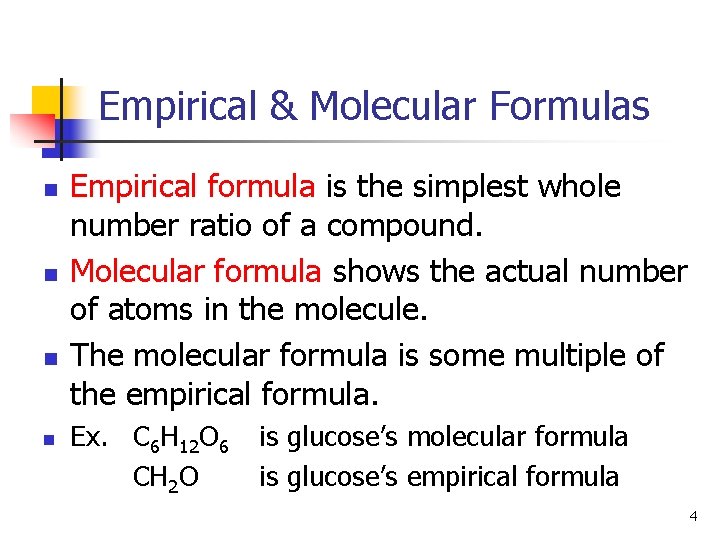
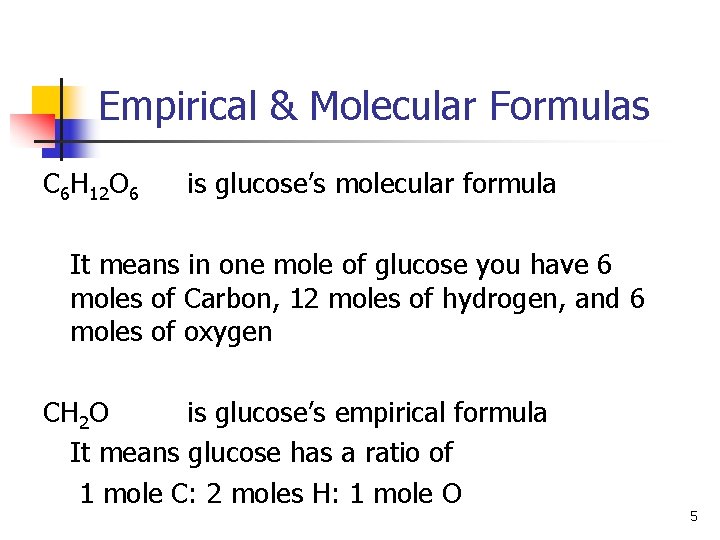
Empirical & Molecular Formulas n n Empirical formula is the simplest whole number ratio of a compound. Molecular formula shows the actual number of atoms in the molecule. The molecular formula is some multiple of the empirical formula. Ex. C 6 H 12 O 6 CH 2 O is glucose’s molecular formula is glucose’s empirical formula 4

Empirical & Molecular Formulas C 6 H 12 O 6 is glucose’s molecular formula It means in one mole of glucose you have 6 moles of Carbon, 12 moles of hydrogen, and 6 moles of oxygen CH 2 O is glucose’s empirical formula It means glucose has a ratio of 1 mole C: 2 moles H: 1 mole O 5

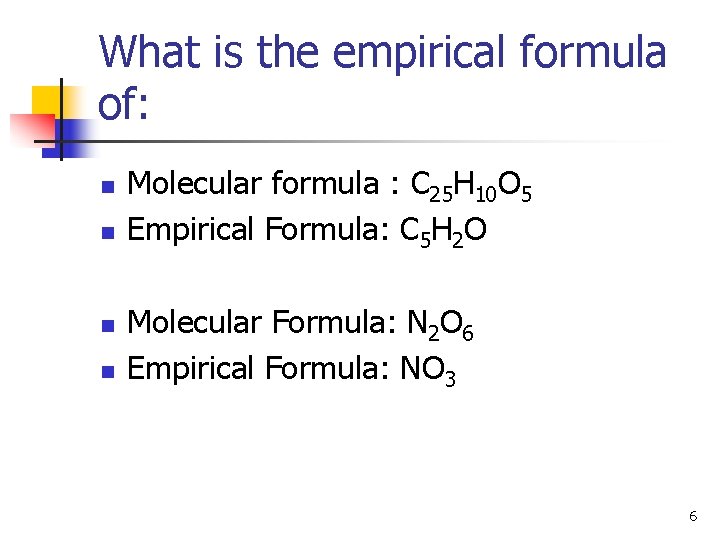
What is the empirical formula of: n n Molecular formula : C 25 H 10 O 5 Empirical Formula: C 5 H 2 O Molecular Formula: N 2 O 6 Empirical Formula: NO 3 6

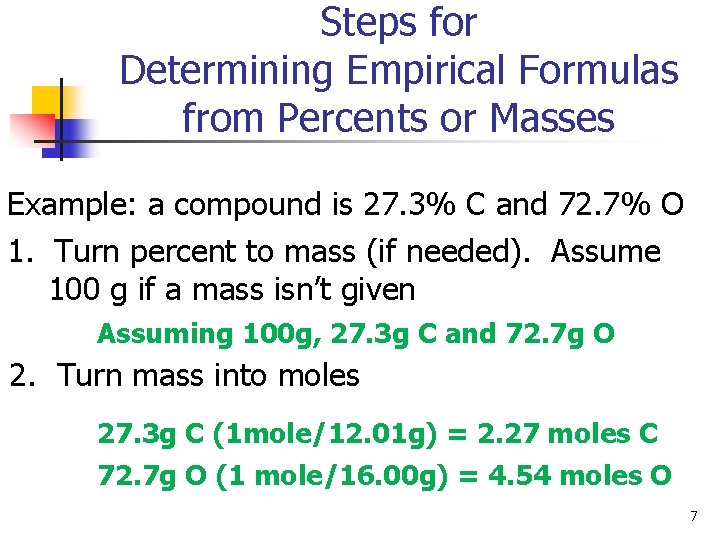
Steps for Determining Empirical Formulas from Percents or Masses Example: a compound is 27. 3% C and 72. 7% O 1. Turn percent to mass (if needed). Assume 100 g if a mass isn’t given Assuming 100 g, 27. 3 g C and 72. 7 g O 2. Turn mass into moles 27. 3 g C (1 mole/12. 01 g) = 2. 27 moles C 72. 7 g O (1 mole/16. 00 g) = 4. 54 moles O 7

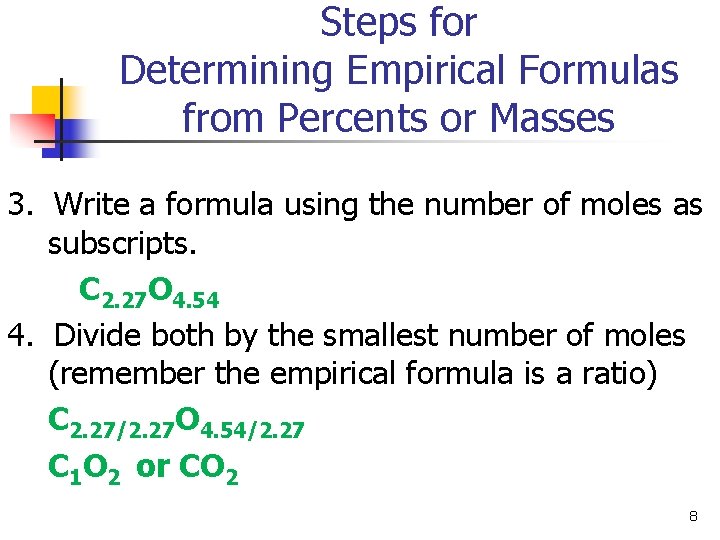
Steps for Determining Empirical Formulas from Percents or Masses 3. Write a formula using the number of moles as subscripts. C 2. 27 O 4. 54 4. Divide both by the smallest number of moles (remember the empirical formula is a ratio) C 2. 27/2. 27 O 4. 54/2. 27 C 1 O 2 or CO 2 8

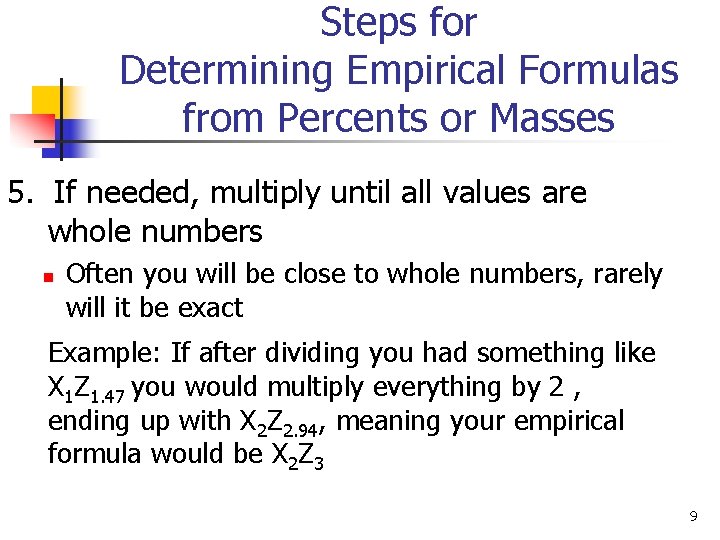
Steps for Determining Empirical Formulas from Percents or Masses 5. If needed, multiply until all values are whole numbers n Often you will be close to whole numbers, rarely will it be exact Example: If after dividing you had something like X 1 Z 1. 47 you would multiply everything by 2 , ending up with X 2 Z 2. 94, meaning your empirical formula would be X 2 Z 3 9

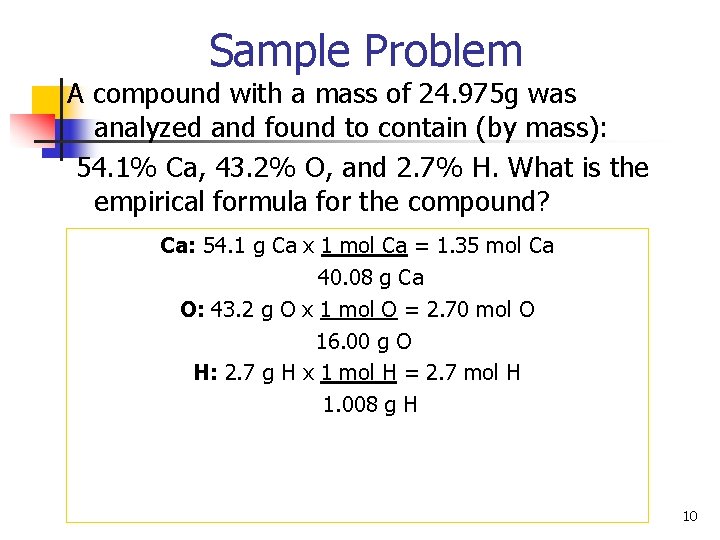
Sample Problem A compound with a mass of 24. 975 g was analyzed and found to contain (by mass): 54. 1% Ca, 43. 2% O, and 2. 7% H. What is the empirical formula for the compound? Ca: 54. 1 g Ca x 1 mol Ca = 1. 35 mol Ca 40. 08 g Ca O: 43. 2 g O x 1 mol O = 2. 70 mol O 16. 00 g O H: 2. 7 g H x 1 mol H = 2. 7 mol H 1. 008 g H 10

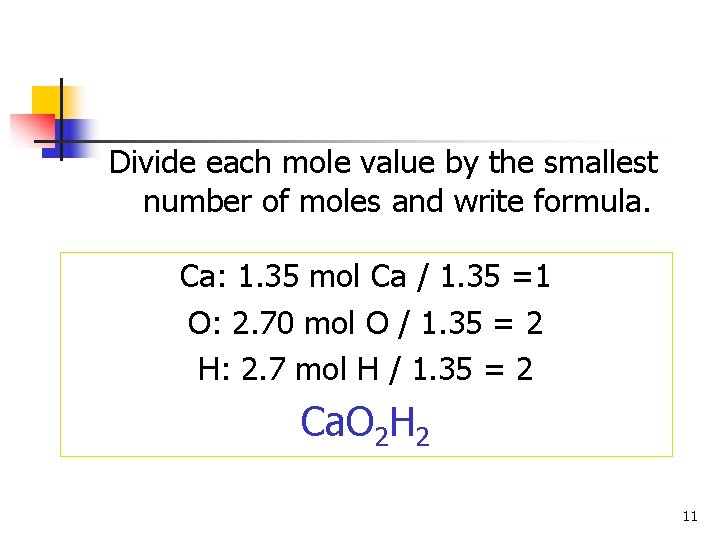
Divide each mole value by the smallest number of moles and write formula. Ca: 1. 35 mol Ca / 1. 35 =1 O: 2. 70 mol O / 1. 35 = 2 H: 2. 7 mol H / 1. 35 = 2 Ca. O 2 H 2 11

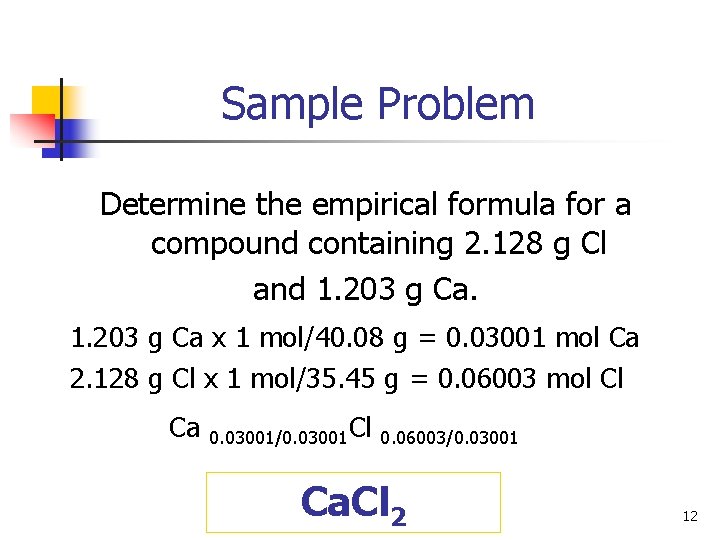
Sample Problem Determine the empirical formula for a compound containing 2. 128 g Cl and 1. 203 g Ca x 1 mol/40. 08 g = 0. 03001 mol Ca 2. 128 g Cl x 1 mol/35. 45 g = 0. 06003 mol Cl Ca 0. 03001/0. 03001 Cl 0. 06003/0. 03001 Ca. Cl 2 12

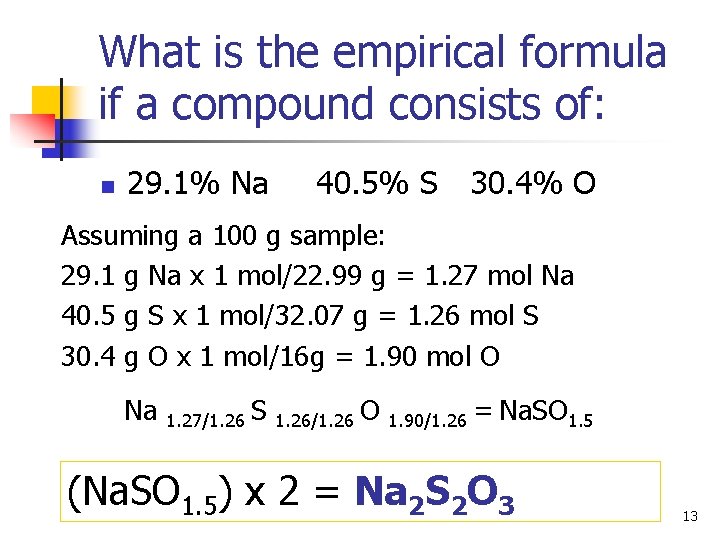
What is the empirical formula if a compound consists of: n 29. 1% Na 40. 5% S 30. 4% O Assuming a 100 g sample: 29. 1 g Na x 1 mol/22. 99 g = 1. 27 mol Na 40. 5 g S x 1 mol/32. 07 g = 1. 26 mol S 30. 4 g O x 1 mol/16 g = 1. 90 mol O Na 1. 27/1. 26 S 1. 26/1. 26 O 1. 90/1. 26 = Na. SO 1. 5 (Na. SO 1. 5) x 2 = Na 2 S 2 O 3 13

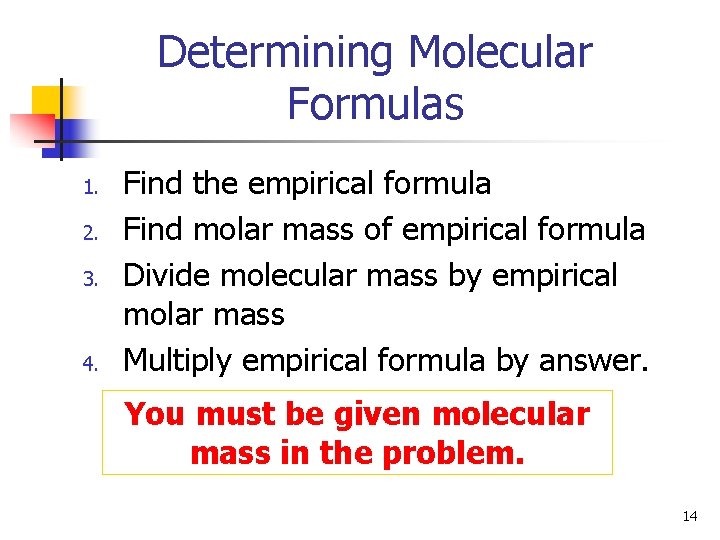
Determining Molecular Formulas 1. 2. 3. 4. Find the empirical formula Find molar mass of empirical formula Divide molecular mass by empirical molar mass Multiply empirical formula by answer. You must be given molecular mass in the problem. 14

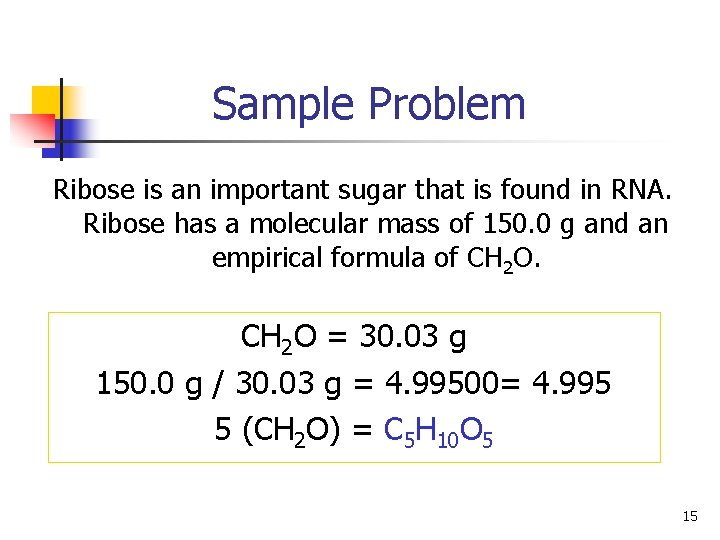
Sample Problem Ribose is an important sugar that is found in RNA. Ribose has a molecular mass of 150. 0 g and an empirical formula of CH 2 O = 30. 03 g 150. 0 g / 30. 03 g = 4. 99500= 4. 995 5 (CH 2 O) = C 5 H 10 O 5 15

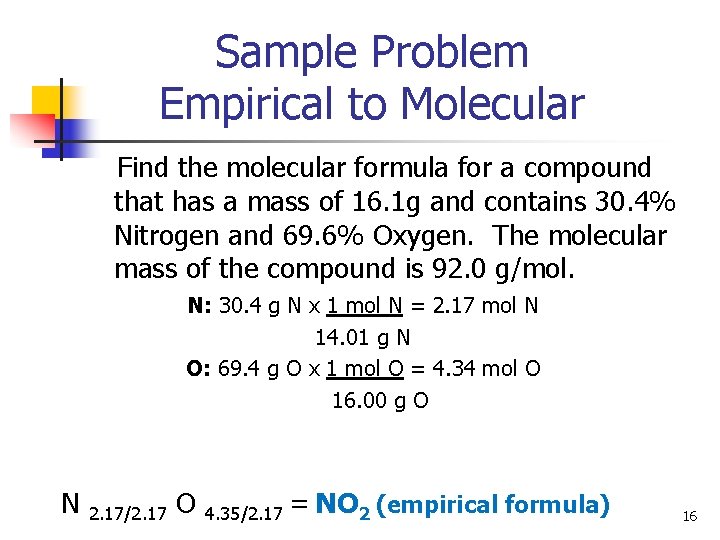
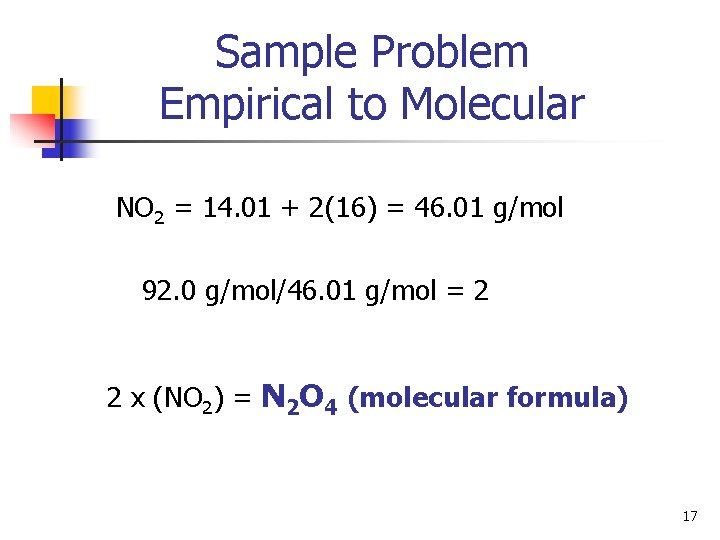
Sample Problem Empirical to Molecular Find the molecular formula for a compound that has a mass of 16. 1 g and contains 30. 4% Nitrogen and 69. 6% Oxygen. The molecular mass of the compound is 92. 0 g/mol. N: 30. 4 g N x 1 mol N = 2. 17 mol N 14. 01 g N O: 69. 4 g O x 1 mol O = 4. 34 mol O 16. 00 g O N 2. 17/2. 17 O 4. 35/2. 17 = NO 2 (empirical formula) 16

Sample Problem Empirical to Molecular NO 2 = 14. 01 + 2(16) = 46. 01 g/mol 92. 0 g/mol/46. 01 g/mol = 2 2 x (NO 2) = N 2 O 4 (molecular formula) 17