p H p OH calculations Titration Calculations Chemistry
![Drill l Drano is mainly sodium hydroxide. The [OH-] is often around 0. 35 Drill l Drano is mainly sodium hydroxide. The [OH-] is often around 0. 35](https://slidetodoc.com/presentation_image_h2/8b9e7e6f962847f4673a051f2caff1e5/image-2.jpg)






- Slides: 14

p. H, p. OH calculations Titration Calculations Chemistry 5/29/15
![Drill l Drano is mainly sodium hydroxide The OH is often around 0 35 Drill l Drano is mainly sodium hydroxide. The [OH-] is often around 0. 35](https://slidetodoc.com/presentation_image_h2/8b9e7e6f962847f4673a051f2caff1e5/image-2.jpg)
Drill l Drano is mainly sodium hydroxide. The [OH-] is often around 0. 35 M. Calculate the p. H, p. OH, and [H+] HW: Finish Acids and Bases Review #35 -37 (I’ll check the whole thing on Monday)

Objectives IWBAT: Calculate the p. H and p. OH of a solution using p. H scale calculations Calculate the concentration of an unknown acid by using titration l NOTES: • Titration Lab– 6/2 & 6/3—Tuesday and Wednesday – CLOSED TOE SHOES • Acids & Bases Test – Friday 6/5

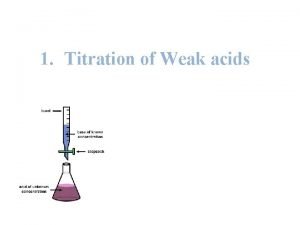
Titration Notes/Demo

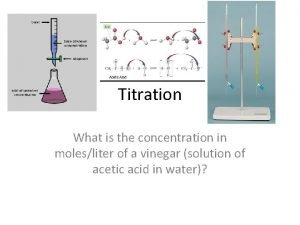
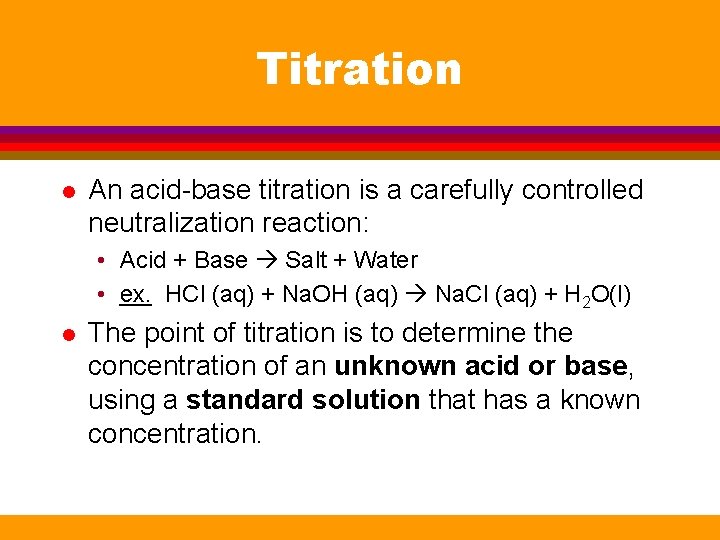
Titration l An acid-base titration is a carefully controlled neutralization reaction: • Acid + Base Salt + Water • ex. HCl (aq) + Na. OH (aq) Na. Cl (aq) + H 2 O(l) l The point of titration is to determine the concentration of an unknown acid or base, using a standard solution that has a known concentration.

Goal of Titration l Procedure used to determine the concentration of an unknown acid or base l Standard solution – solution with the known concentration

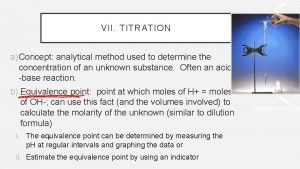
More about Titration l l The equivalence point is when all of the acid and base have neutralized each other. The end point is when the indicator changes color. They should be close together – Why? Some indicators change at high p. H, some at low p. H.

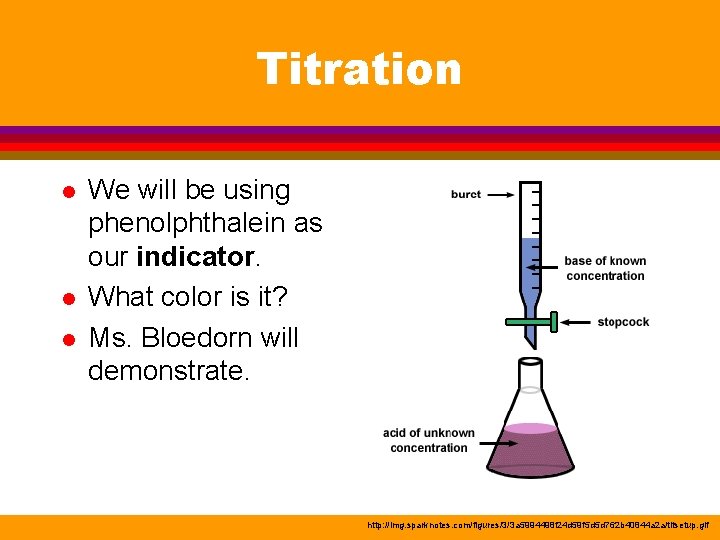
Titration l l l We will be using phenolphthalein as our indicator. What color is it? Ms. Bloedorn will demonstrate. http: //img. sparknotes. com/figures/3/3 a 5994498 f 24 d 59 f 5 d 5 d 762 b 40844 a 2 a/titsetup. gif

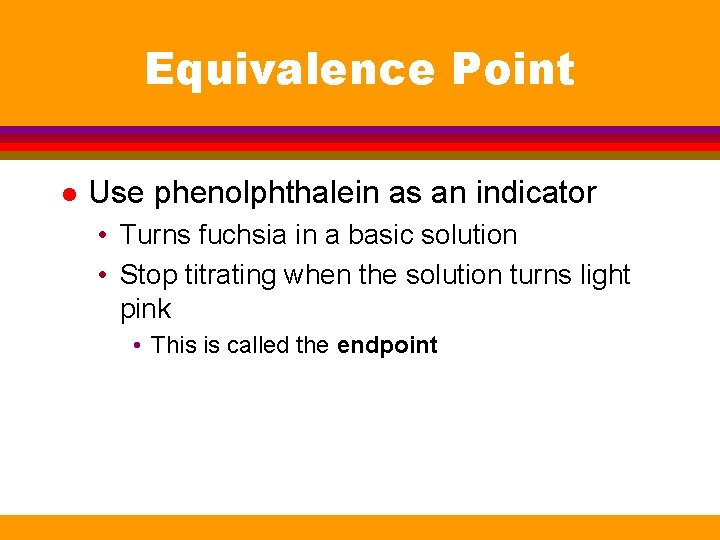
Equivalence Point l Use phenolphthalein as an indicator • Turns fuchsia in a basic solution • Stop titrating when the solution turns light pink • This is called the endpoint

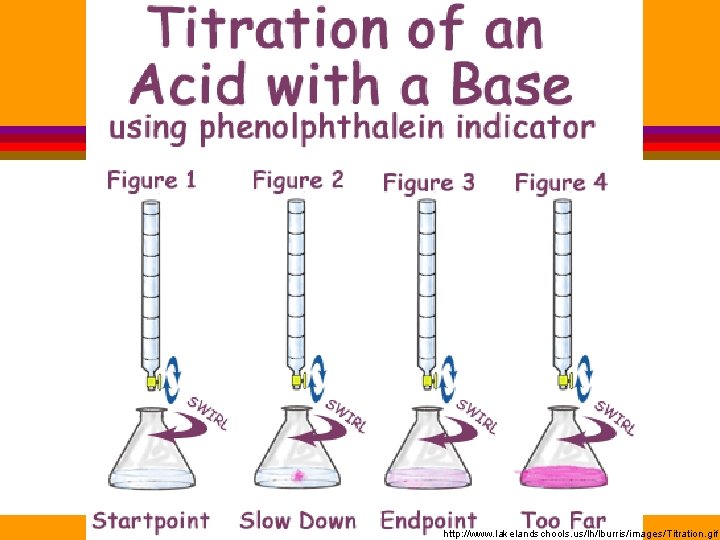
http: //www. lakelandschools. us/lh/lburris/images/Titration. gif


Titration Calculations l l l Let’s go through Scenario #1 together. Do Scenario #2 on your own. Let’s review

Titration Practice Problems l l l Work on the Titration Problems Make sure to show the mole ratio step! I’ll circulate to help—check the answer key as you go

Closure l What is the point of a titration? l What is the difference between “end point” and “equivalence point”?
 Titration vs back titration
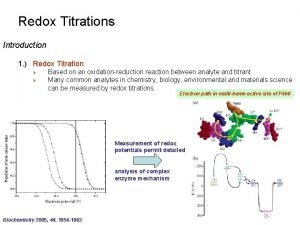
Titration vs back titration Introduction to redox titration
Introduction to redox titration Acid base titration vs redox titration
Acid base titration vs redox titration Titration vs back titration
Titration vs back titration Titration formula
Titration formula Precipitation titration curve
Precipitation titration curve Back titration calculations
Back titration calculations Titration calculations
Titration calculations Titration formula
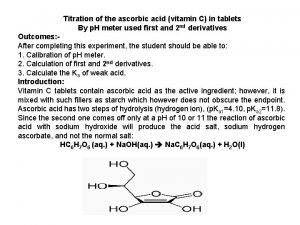
Titration formula Ascorbic acid titration curve
Ascorbic acid titration curve Types of connections in steel structures
Types of connections in steel structures Urine anion gap calculator
Urine anion gap calculator Ib chemistry functional groups
Ib chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry Permanganometry principle
Permanganometry principle