OxidationReduction Dr Ron Rusay Fall 2001 Copyright 2001



















- Slides: 33

Oxidation-Reduction Dr. Ron Rusay Fall 2001 © Copyright 2001 R. J. Rusay

Oxidation-Reduction • Oxidation is the loss of electrons. • Reduction is the gain of electrons. • The reactions occur together. One does not occur without the other. • The terms are used relative to the change in the oxidation state of the reactant(s).

Oxidation Reduction Reactions

Oxidation State (Oxidation Number)


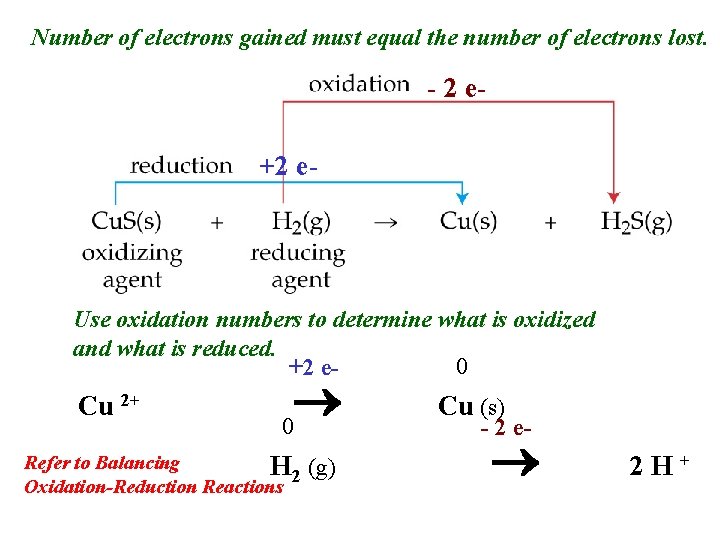
Number of electrons gained must equal the number of electrons lost. - 2 e+2 e- Use oxidation numbers to determine what is oxidized and what is reduced. 0 +2 e- Cu 2+ 0 Refer to Balancing H 2 Oxidation-Reduction Reactions (g) Cu (s) - 2 e- 2 H+

Balancing Redox Equations in acidic solutions 1) Determine the oxidation numbers of atoms in both reactants and products. 2) Identify and select out those which change oxidation number (“redox” atoms) into separate “half reactions”. 3) Balance the “redox” atoms and charges (electron gain and loss must equal!). 4) In acidic reactions balance oxygen with water then hydrogen from water with acid proton(s). © Copyright 1995 - 2001 R. J. Rusay

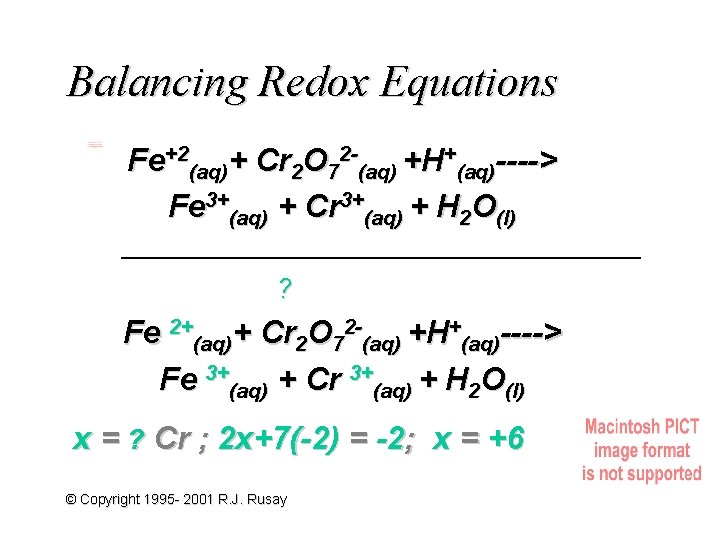
Balancing Redox Equations Fe+2(aq)+ Cr 2 O 72 -(aq) +H+(aq)----> Fe 3+(aq) + Cr 3+(aq) + H 2 O(l) ? Fe 2+(aq)+ Cr 2 O 72 -(aq) +H+(aq)----> Fe 3+(aq) + Cr 3+(aq) + H 2 O(l) x = ? Cr ; 2 x+7(-2) = -2; x = +6 © Copyright 1995 - 2001 R. J. Rusay

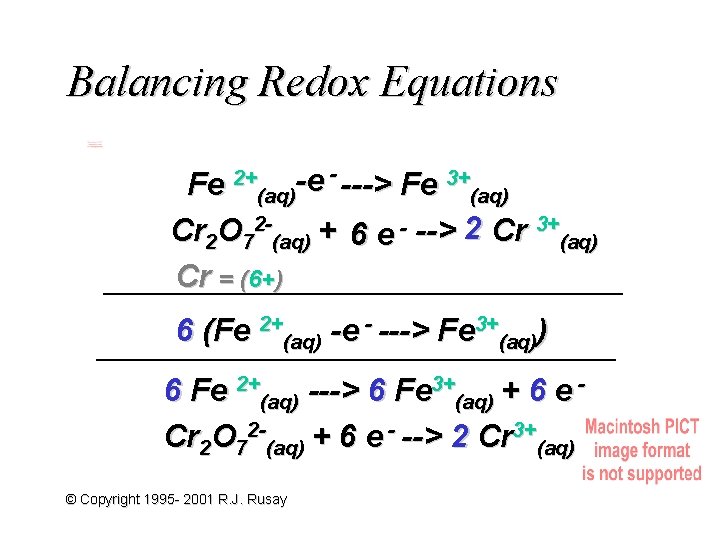
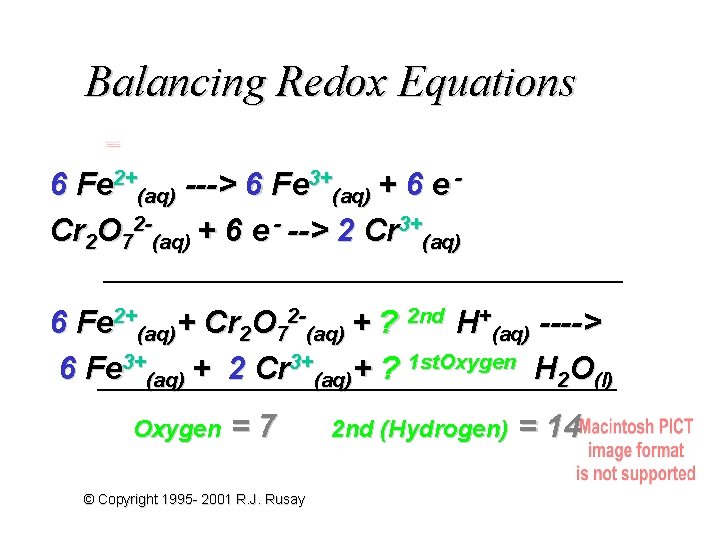
Balancing Redox Equations Fe 2+(aq)-e - ---> Fe 3+(aq) Cr 2 O 72 -(aq) + 6 e - --> 2 Cr 3+(aq) Cr = (6+) 6 (Fe 2+(aq) -e - ---> Fe 3+(aq)) 6 Fe 2+(aq) ---> 6 Fe 3+(aq) + 6 e Cr 2 O 72 -(aq) + 6 e - --> 2 Cr 3+(aq) © Copyright 1995 - 2001 R. J. Rusay

Balancing Redox Equations 6 Fe 2+(aq) ---> 6 Fe 3+(aq) + 6 e Cr 2 O 72 -(aq) + 6 e - --> 2 Cr 3+(aq) 6 Fe 2+(aq)+ Cr 2 O 72 -(aq) + ? 2 nd H+(aq) ----> 6 Fe 3+(aq) + 2 Cr 3+(aq)+ ? 1 st. Oxygen H 2 O(l) Oxygen =7 © Copyright 1995 - 2001 R. J. Rusay 2 nd (Hydrogen) = 14

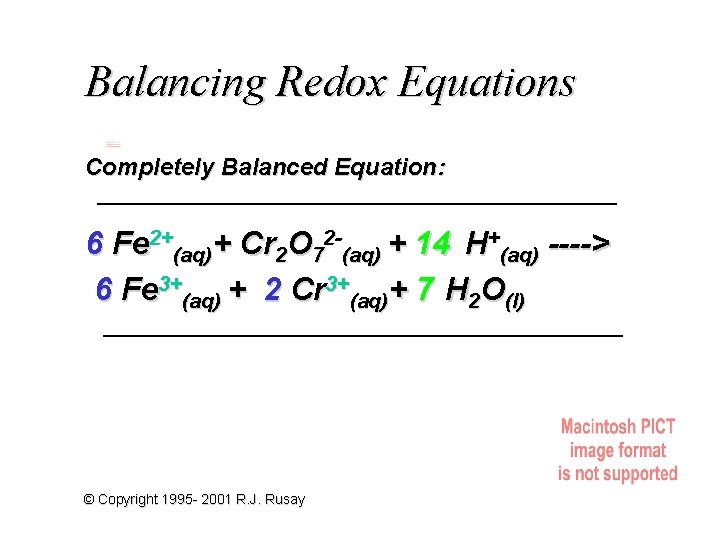
Balancing Redox Equations Completely Balanced Equation: 6 Fe 2+(aq)+ Cr 2 O 72 -(aq) + 14 H+(aq) ----> 6 Fe 3+(aq) + 2 Cr 3+(aq)+ 7 H 2 O(l) © Copyright 1995 - 2001 R. J. Rusay

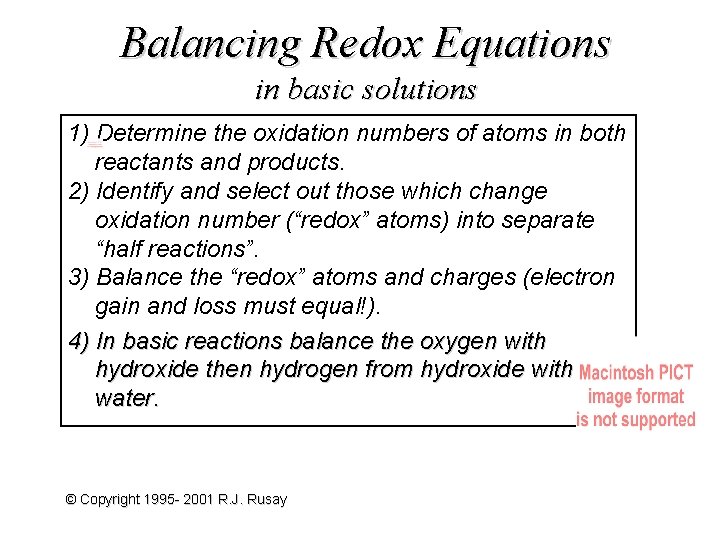
Balancing Redox Equations in basic solutions 1) Determine the oxidation numbers of atoms in both reactants and products. 2) Identify and select out those which change oxidation number (“redox” atoms) into separate “half reactions”. 3) Balance the “redox” atoms and charges (electron gain and loss must equal!). 4) In basic reactions balance the oxygen with hydroxide then hydrogen from hydroxide with water. © Copyright 1995 - 2001 R. J. Rusay


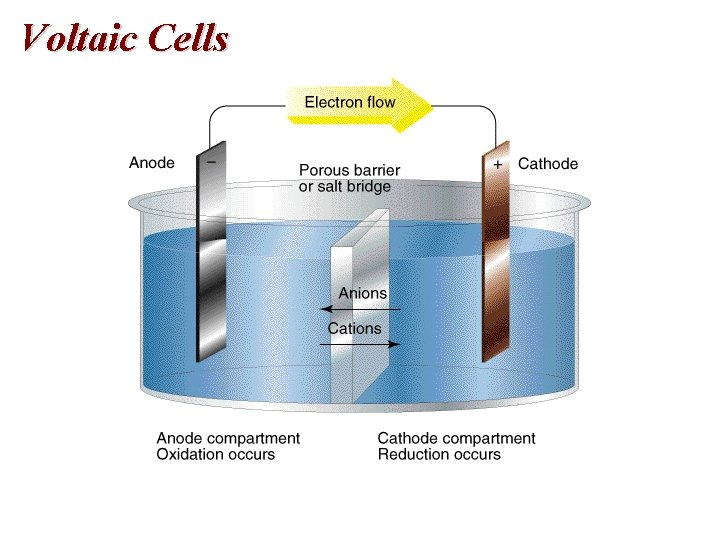
A Voltaic Cell

Voltaic Cells


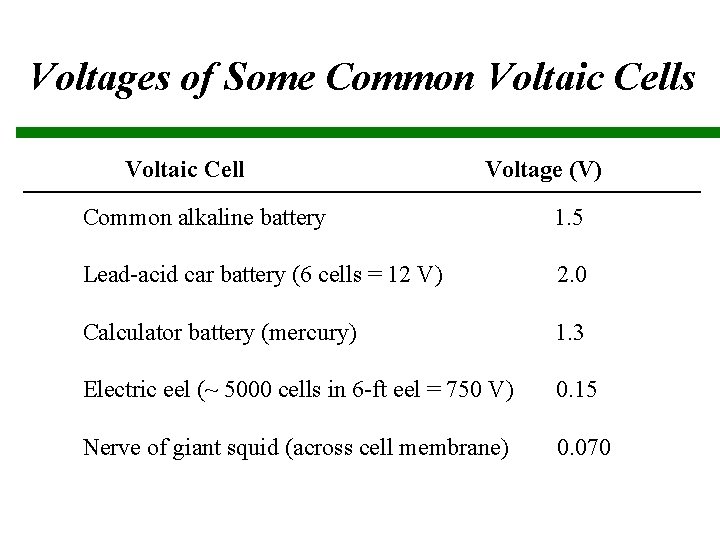
Voltages of Some Common Voltaic Cells Voltaic Cell Voltage (V) Common alkaline battery 1. 5 Lead-acid car battery (6 cells = 12 V) 2. 0 Calculator battery (mercury) 1. 3 Electric eel (~ 5000 cells in 6 -ft eel = 750 V) 0. 15 Nerve of giant squid (across cell membrane) 0. 070


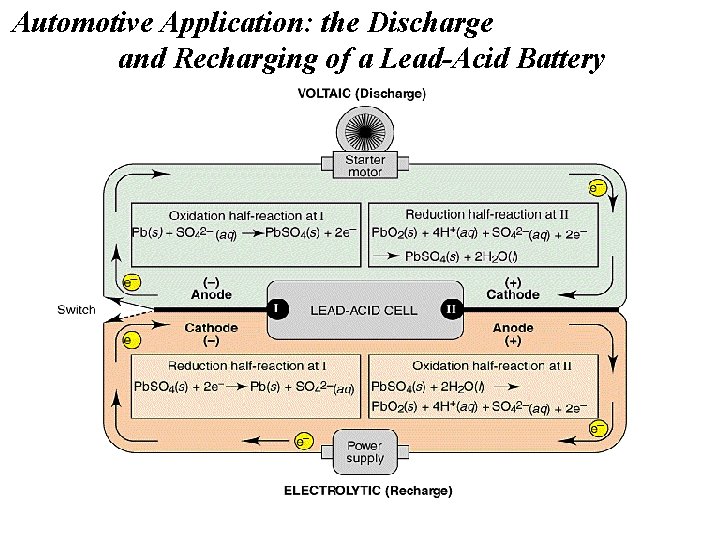
Automotive Application: the Discharge and Recharging of a Lead-Acid Battery

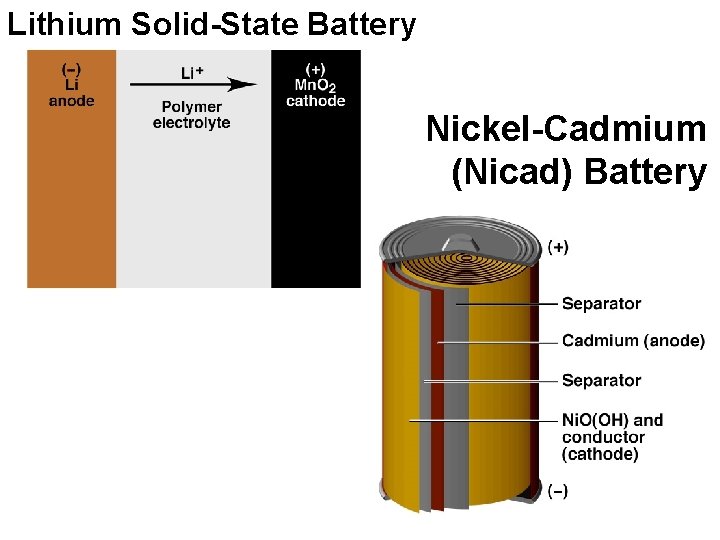
Lithium Solid-State Battery Nickel-Cadmium (Nicad) Battery

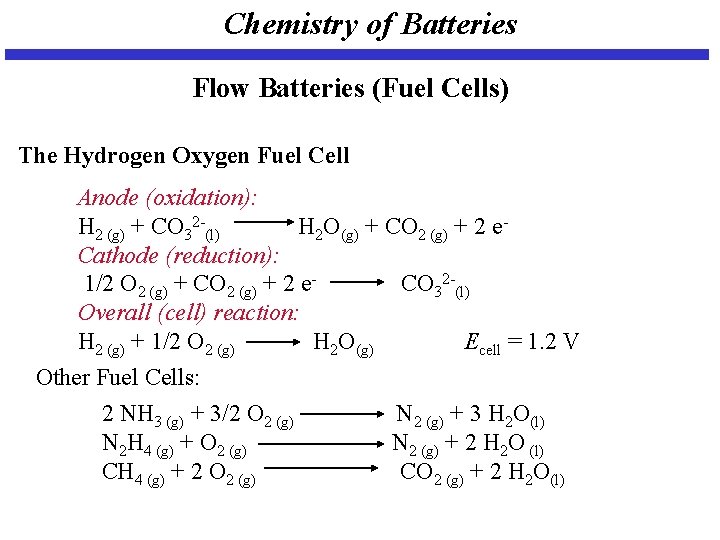
Chemistry of Batteries Flow Batteries (Fuel Cells) The Hydrogen Oxygen Fuel Cell Anode (oxidation): H 2 (g) + CO 32 -(l) H 2 O(g) + CO 2 (g) + 2 e. Cathode (reduction): 1/2 O 2 (g) + CO 2 (g) + 2 e. CO 32 -(l) Overall (cell) reaction: H 2 (g) + 1/2 O 2 (g) H 2 O(g) Ecell = 1. 2 V Other Fuel Cells: 2 NH 3 (g) + 3/2 O 2 (g) N 2 (g) + 3 H 2 O(l) N 2 H 4 (g) + O 2 (g) N 2 (g) + 2 H 2 O (l) CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O(l)







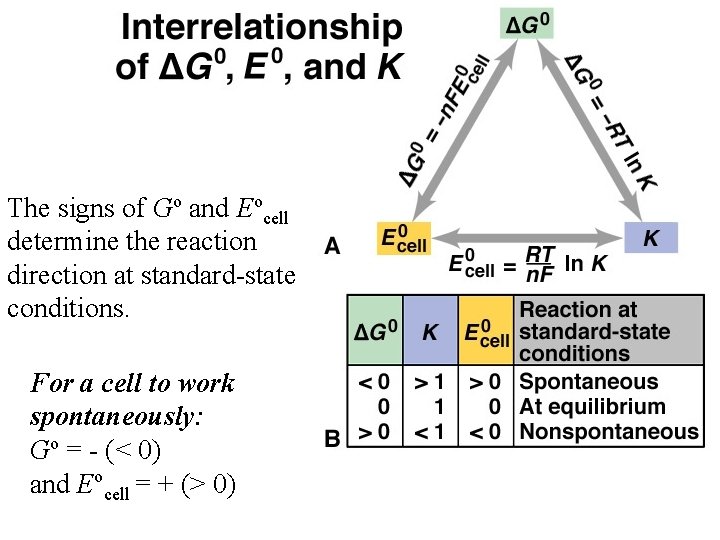
The signs of Go and Eocell determine the reaction direction at standard-state conditions. For a cell to work spontaneously: Go = - (< 0) and Eocell = + (> 0)

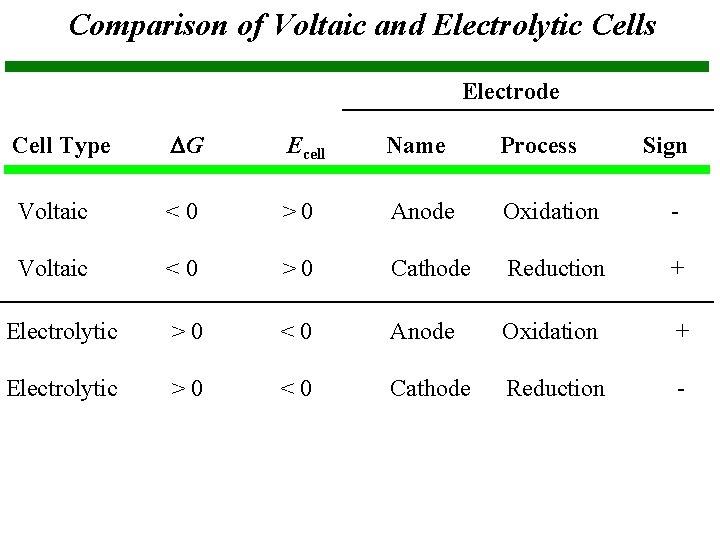
Comparison of Voltaic and Electrolytic Cells Electrode Cell Type G Ecell Name Process Voltaic <0 >0 Anode Oxidation - Voltaic <0 >0 Cathode Reduction + Electrolytic >0 <0 Anode Oxidation + Electrolytic >0 <0 Cathode Reduction - Sign

Electrochemical Reactions

Electrolysis: Chrome Plating

Aluminum Production Using an Electrolytic Cell Do you recycle? It takes only 5% of the energy to produce a can from recycled aluminum vs. aluminum ore.
