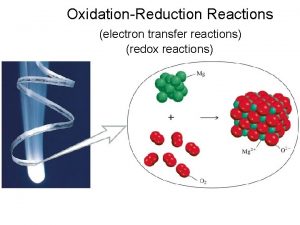
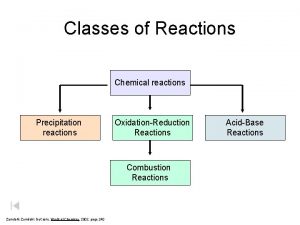
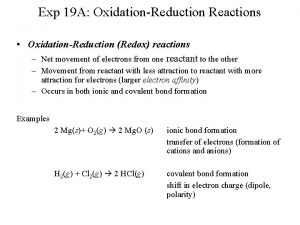
OxidationReduction Reactions Oxidationreduction reaction a chemical reaction involving



- Slides: 20

Oxidation-Reduction Reactions • Oxidation-reduction reaction – a chemical reaction involving the transfer of electrons – Oxidation – loss of electrons – Reduction – gain of electrons

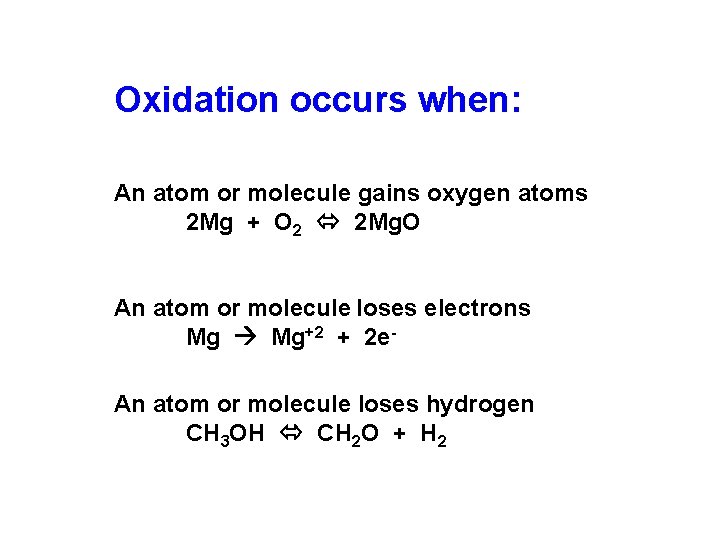
Oxidation occurs when: An atom or molecule gains oxygen atoms 2 Mg + O 2 2 Mg. O An atom or molecule loses electrons Mg Mg+2 + 2 e. An atom or molecule loses hydrogen CH 3 OH CH 2 O + H 2

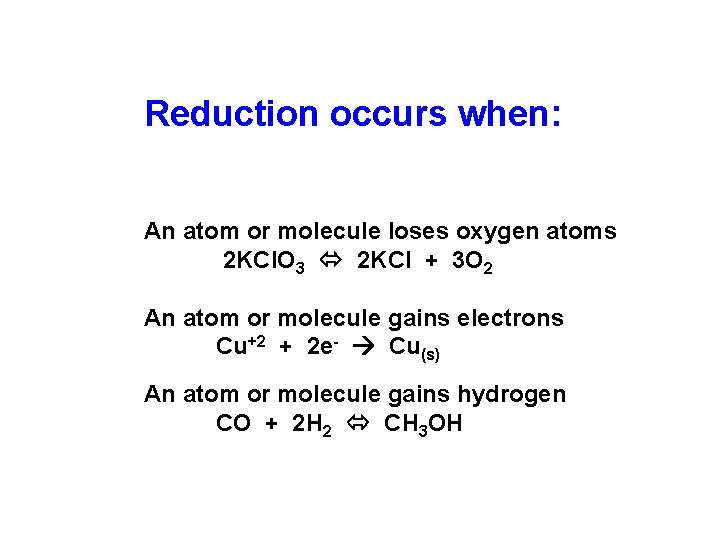
Reduction occurs when: An atom or molecule loses oxygen atoms 2 KCl. O 3 2 KCl + 3 O 2 An atom or molecule gains electrons Cu+2 + 2 e- Cu(s) An atom or molecule gains hydrogen CO + 2 H 2 CH 3 OH

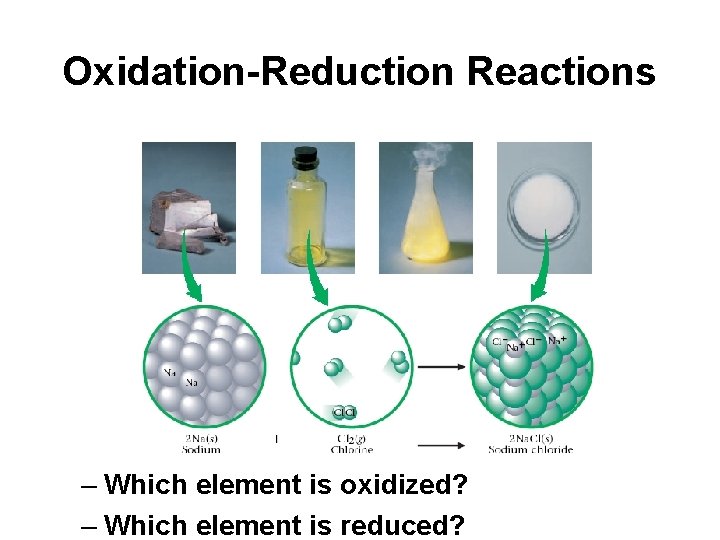
Oxidation-Reduction Reactions – Which element is oxidized? – Which element is reduced?

• Assignment: • Page 651/ section 18 -2: 1 -7

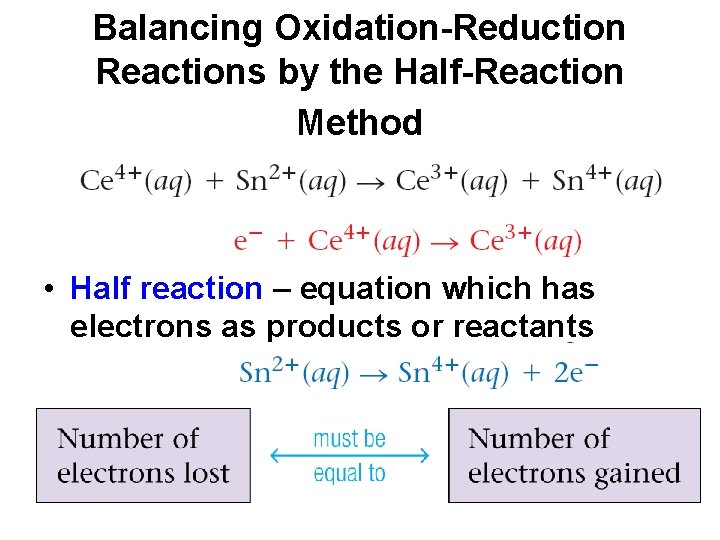
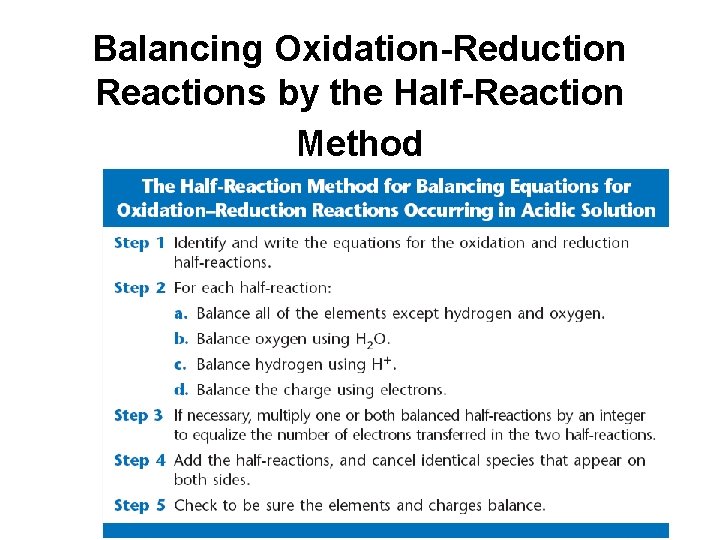
Balancing Oxidation-Reduction Reactions by the Half-Reaction Method • Half reaction – equation which has electrons as products or reactants

Balancing Oxidation-Reduction Reactions by the Half-Reaction Method

Electrochemistry: An Introduction • Electrochemistry – the study of the interchange of chemical and electrical energy • Two types of processes – Production of an electric current from a chemical reaction – The use of electric current to produce chemical change

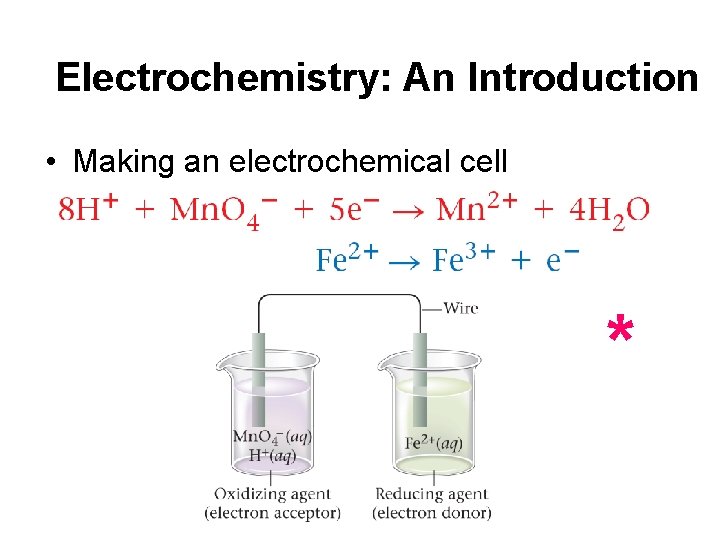
Electrochemistry: An Introduction • Making an electrochemical cell *

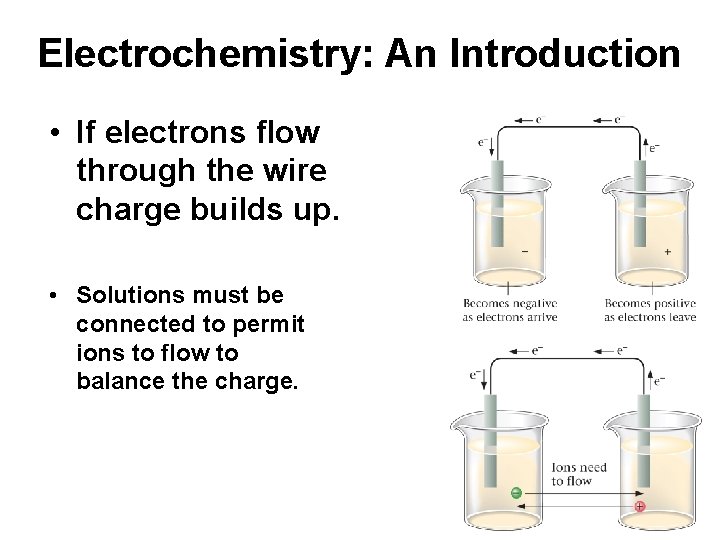
Electrochemistry: An Introduction • If electrons flow through the wire charge builds up. • Solutions must be connected to permit ions to flow to balance the charge.

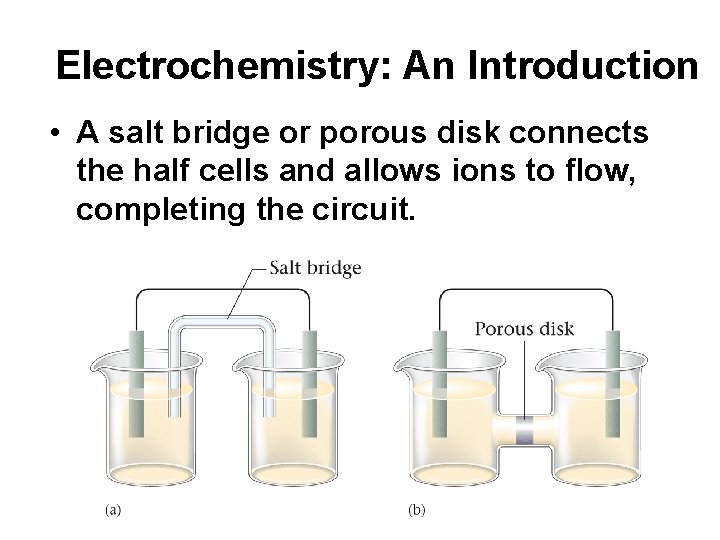
Electrochemistry: An Introduction • A salt bridge or porous disk connects the half cells and allows ions to flow, completing the circuit.

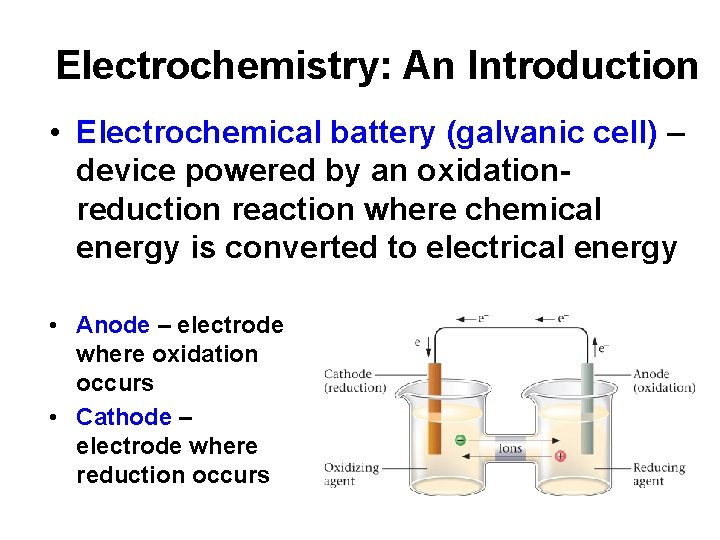
Electrochemistry: An Introduction • Electrochemical battery (galvanic cell) – device powered by an oxidationreduction reaction where chemical energy is converted to electrical energy • Anode – electrode where oxidation occurs • Cathode – electrode where reduction occurs

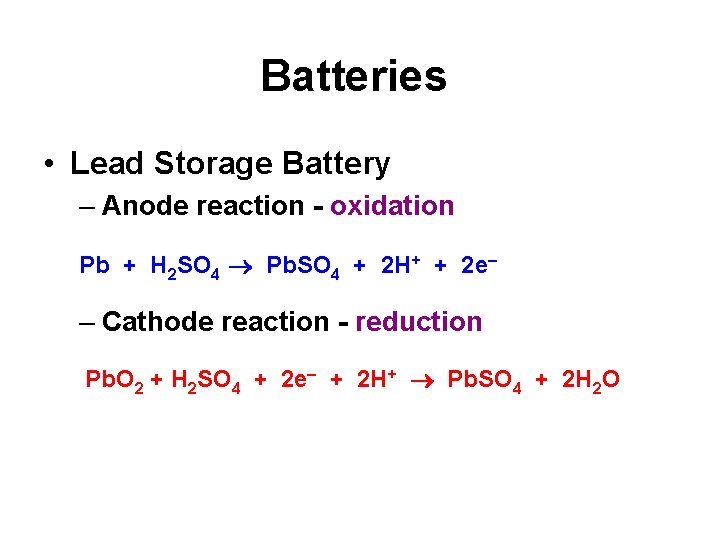
Batteries • Lead Storage Battery – Anode reaction - oxidation Pb + H 2 SO 4 Pb. SO 4 + 2 H+ + 2 e – Cathode reaction - reduction Pb. O 2 + H 2 SO 4 + 2 e + 2 H+ Pb. SO 4 + 2 H 2 O

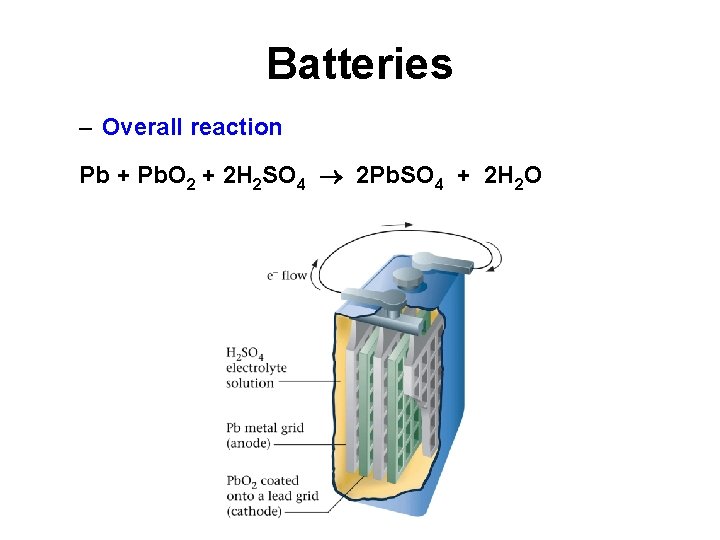
Batteries – Overall reaction Pb + Pb. O 2 + 2 H 2 SO 4 2 Pb. SO 4 + 2 H 2 O

Batteries • Electric Potential – the “pressure” on electrons to flow from anode to cathode in a battery

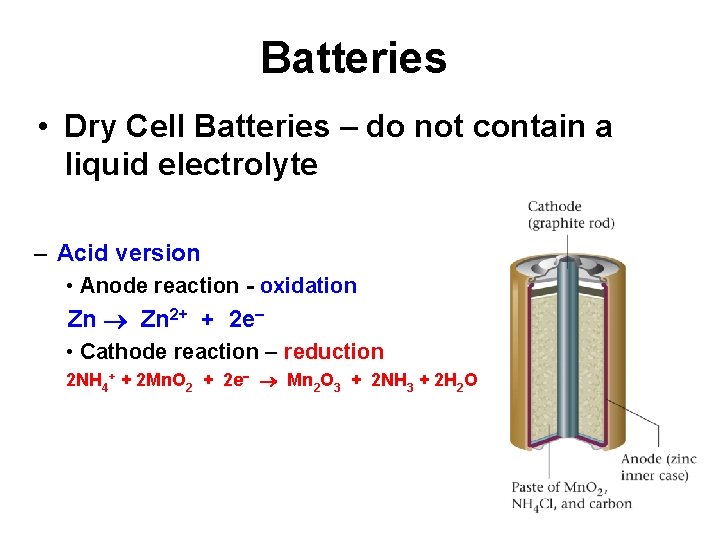
Batteries • Dry Cell Batteries – do not contain a liquid electrolyte – Acid version • Anode reaction - oxidation Zn 2+ + 2 e • Cathode reaction – reduction 2 NH 4+ + 2 Mn. O 2 + 2 e Mn 2 O 3 + 2 NH 3 + 2 H 2 O

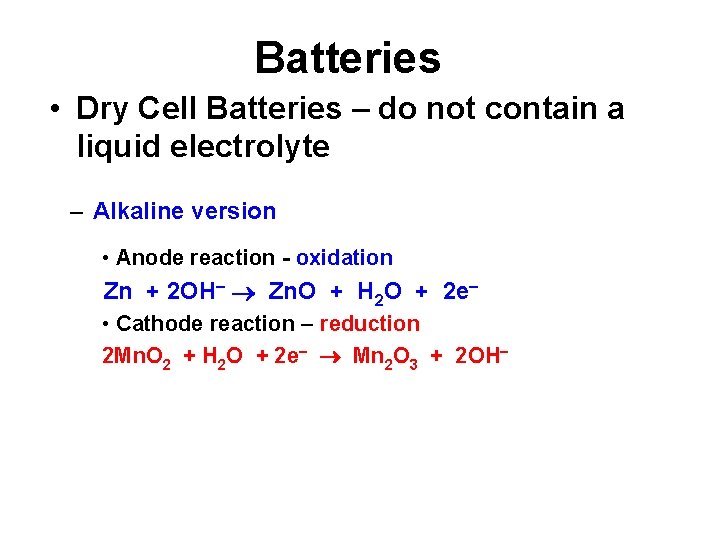
Batteries • Dry Cell Batteries – do not contain a liquid electrolyte – Alkaline version • Anode reaction - oxidation Zn + 2 OH Zn. O + H 2 O + 2 e • Cathode reaction – reduction 2 Mn. O 2 + H 2 O + 2 e Mn 2 O 3 + 2 OH

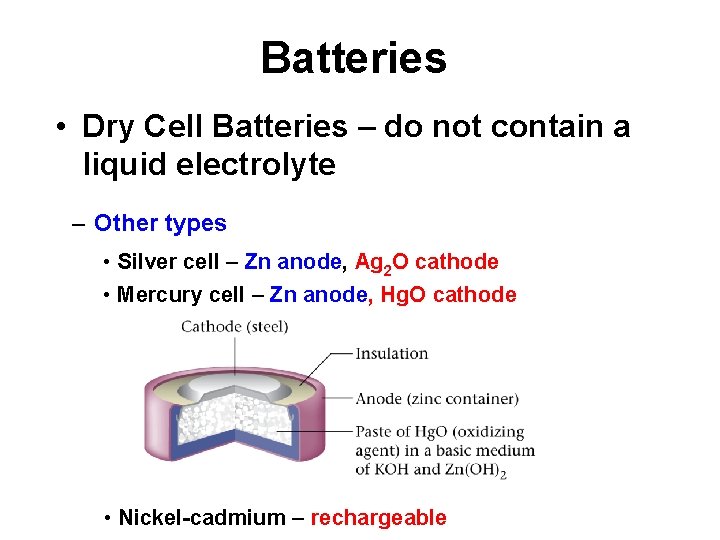
Batteries • Dry Cell Batteries – do not contain a liquid electrolyte – Other types • Silver cell – Zn anode, Ag 2 O cathode • Mercury cell – Zn anode, Hg. O cathode • Nickel-cadmium – rechargeable

Corrosion • Corrosion is the oxidation of metals to form mainly oxides and sulfides. – Some metals, such as aluminum, protect themselves with their oxide coating. – Corrosion of iron can be prevented by coatings, by alloying and cathodic protection. * Cathodic protection of an underground pipe

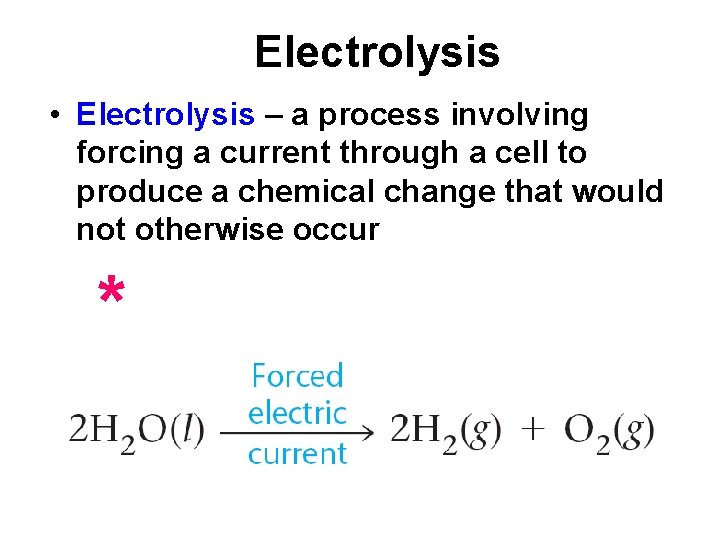
Electrolysis • Electrolysis – a process involving forcing a current through a cell to produce a chemical change that would not otherwise occur *
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Types of reactions
Types of reactions Are kc and kp equal
Are kc and kp equal Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Enzyme activity graph
Enzyme activity graph Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Balance redox half reactions
Balance redox half reactions Bomb power
Bomb power Indications of chemical reactions
Indications of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions Solvent in chemical reactions
Solvent in chemical reactions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Examples of double replacement reactions
Examples of double replacement reactions Jared investigated chemical reactions
Jared investigated chemical reactions How to identify type of reaction
How to identify type of reaction Equilibrium of chemical reactions
Equilibrium of chemical reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Are all chemical reactions reversible
Are all chemical reactions reversible Chemistry unit 4 grade 11
Chemistry unit 4 grade 11 Four types of chemical reactions
Four types of chemical reactions