Organic Chemistry continued Alkenes Alkenes General formula Cn

- Slides: 10

Organic Chemistry – continued Alkenes

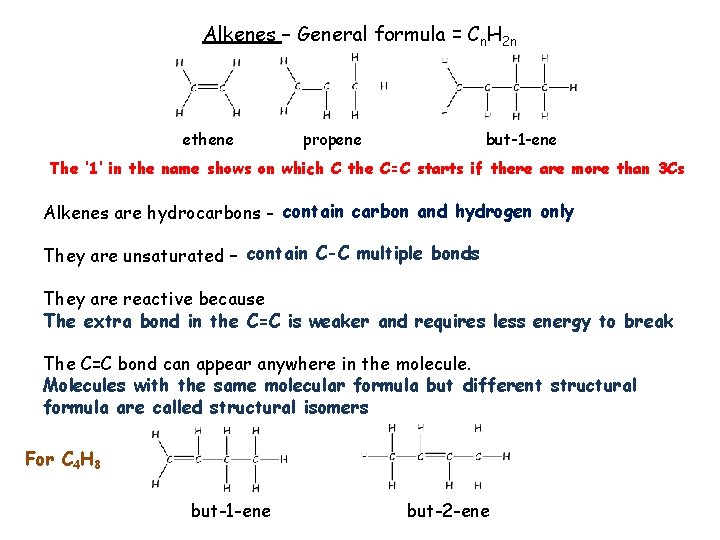
Alkenes – General formula = Cn. H 2 n ethene propene but-1 -ene The ‘ 1’ in the name shows on which C the C=C starts if there are more than 3 Cs Alkenes are hydrocarbons - contain carbon and hydrogen only They are unsaturated – contain C-C multiple bonds They are reactive because The extra bond in the C=C is weaker and requires less energy to break The C=C bond can appear anywhere in the molecule. Molecules with the same molecular formula but different structural formula are called structural isomers For C 4 H 8 but-1 -ene but-2 -ene

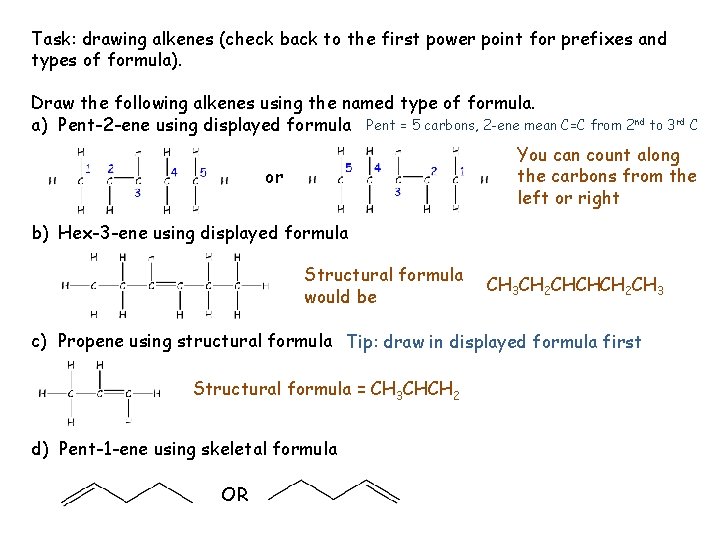
Task: drawing alkenes (check back to the first power point for prefixes and types of formula). Draw the following alkenes using the named type of formula. a) Pent-2 -ene using displayed formula Pent = 5 carbons, 2 -ene mean C=C from 2 nd to 3 rd C You can count along the carbons from the left or right or b) Hex-3 -ene using displayed formula Structural formula would be CH 3 CH 2 CHCHCH 2 CH 3 c) Propene using structural formula Tip: draw in displayed formula first Structural formula = CH 3 CHCH 2 d) Pent-1 -ene using skeletal formula OR

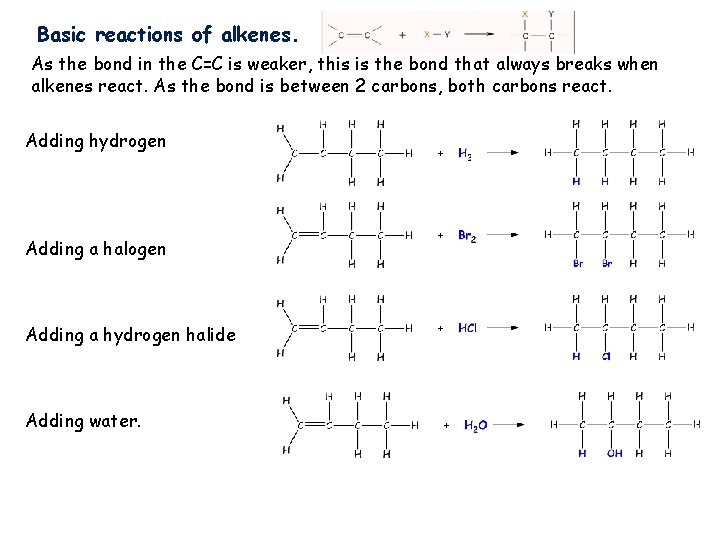
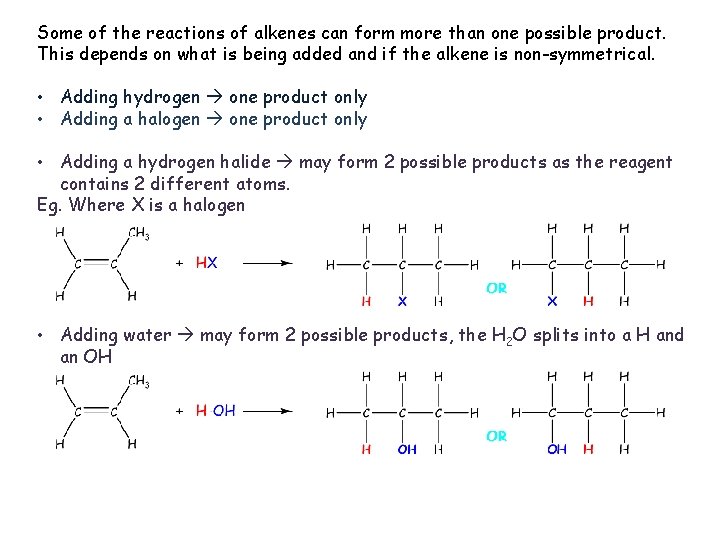
Basic reactions of alkenes. As the bond in the C=C is weaker, this is the bond that always breaks when alkenes react. As the bond is between 2 carbons, both carbons react. Adding hydrogen Adding a halogen Adding a hydrogen halide Adding water.

Some of the reactions of alkenes can form more than one possible product. This depends on what is being added and if the alkene is non-symmetrical. • Adding hydrogen one product only • Adding a halogen one product only • Adding a hydrogen halide may form 2 possible products as the reagent contains 2 different atoms. Eg. Where X is a halogen • Adding water may form 2 possible products, the H 2 O splits into a H and an OH

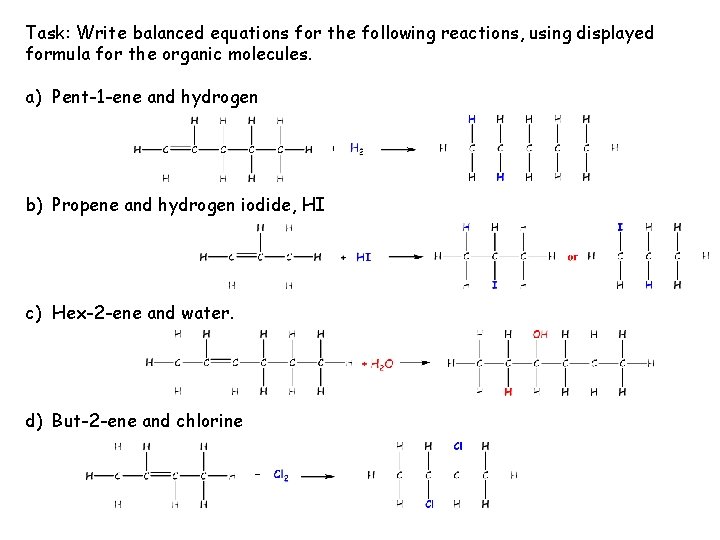
Task: Write balanced equations for the following reactions, using displayed formula for the organic molecules. a) Pent-1 -ene and hydrogen b) Propene and hydrogen iodide, HI c) Hex-2 -ene and water. d) But-2 -ene and chlorine

You can test for the presence of the alkene functional group with one of the reactions on slide 4. To test for a functional group the reaction needs to be able to • Be carried out in a test tube • Give an obvious change that can be seen Of the 4 reactions studied, the reaction that adds a halogen meet these criteria when the halogen is bromine. Watch the video below to see bromine (in the form of bromine water) being added to an alkane (cyclohexane) and an alkene (cyclohexene). https: //www. youtube. com/watch? v=2 C_6 ax 2 Ts. V 8 The reaction uses the fact that bromine water is an orange colour but the product of the reaction is colourless

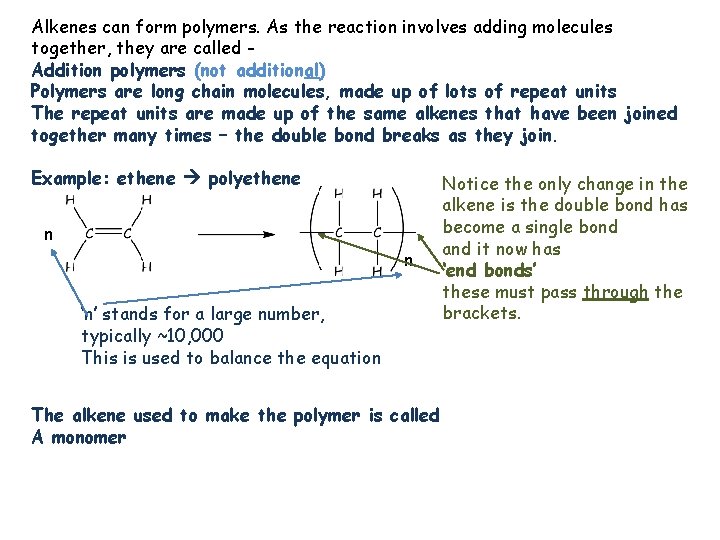
Alkenes can form polymers. As the reaction involves adding molecules together, they are called Addition polymers (not additional) Polymers are long chain molecules, made up of lots of repeat units The repeat units are made up of the same alkenes that have been joined together many times – the double bond breaks as they join. Example: ethene polyethene n n ‘n’ stands for a large number, typically ~10, 000 This is used to balance the equation The alkene used to make the polymer is called A monomer Notice the only change in the alkene is the double bond has become a single bond and it now has ‘end bonds’ these must pass through the brackets.

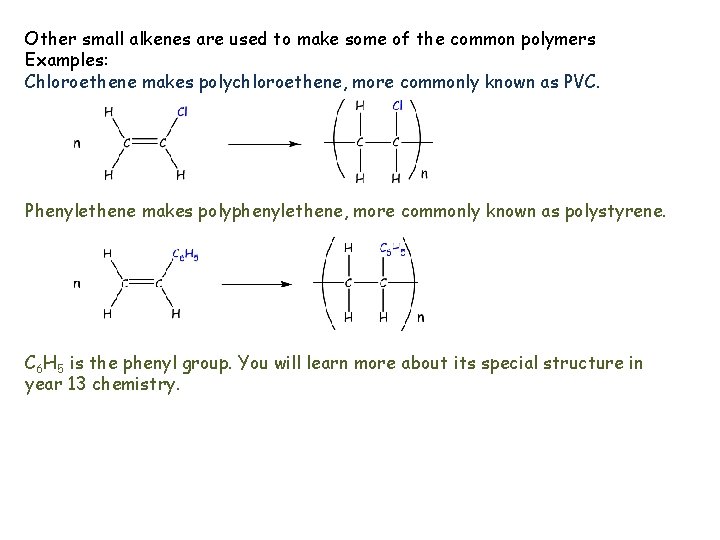
Other small alkenes are used to make some of the common polymers Examples: Chloroethene makes polychloroethene, more commonly known as PVC. Phenylethene makes polyphenylethene, more commonly known as polystyrene. C 6 H 5 is the phenyl group. You will learn more about its special structure in year 13 chemistry.

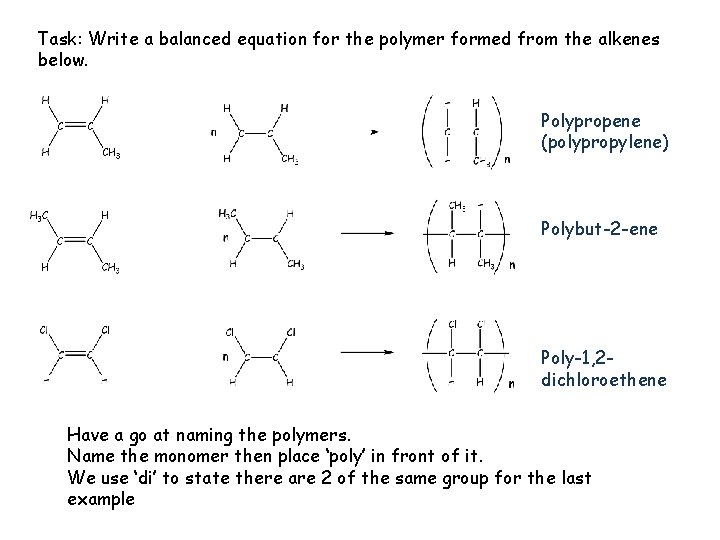
Task: Write a balanced equation for the polymer formed from the alkenes below. Polypropene (polypropylene) Polybut-2 -ene Poly-1, 2 dichloroethene Have a go at naming the polymers. Name the monomer then place ‘poly’ in front of it. We use ‘di’ to state there are 2 of the same group for the last example


















