Office of Partnerships Overview Working Together Works Office






























- Slides: 40

Office of Partnerships Overview Working Together Works

Office of Partnerships Objectives for this presentation: • Review OP’s organizational structure • Provide insight into OP programs and initiatives • Increase understanding of our funding and management capabilities • Review current initiatives • Discuss recommendations for future egg standards Our Goal: • To provide a clear understanding of what OP does and how we do it – through continual dialogue with partners and competent oversight 2

What the Office of Partnerships Does Vision Statement: “A global partnership that protects the public health. ” Mission Statement: “We make partnerships happen through fostering funding opportunities and promoting domestic and international mutual reliance and systems recognition. ” 3

Office of Partnerships Ways we accomplish our mission and protect public health are through funding, promoting national regulatory program standards, analyzing & promoting integration and effectiveness of initiatives: • Contracts, Grants, & Cooperative Agreements • Federal-State Program Policy • Coordination of State Training with Office of Training, Education, & Development (ORA/OTED) • Regulatory Program Standards • Advancing National Integration • Information Sharing • Rapid Response Teams • Food Protection Task Forces • International Engagement 4

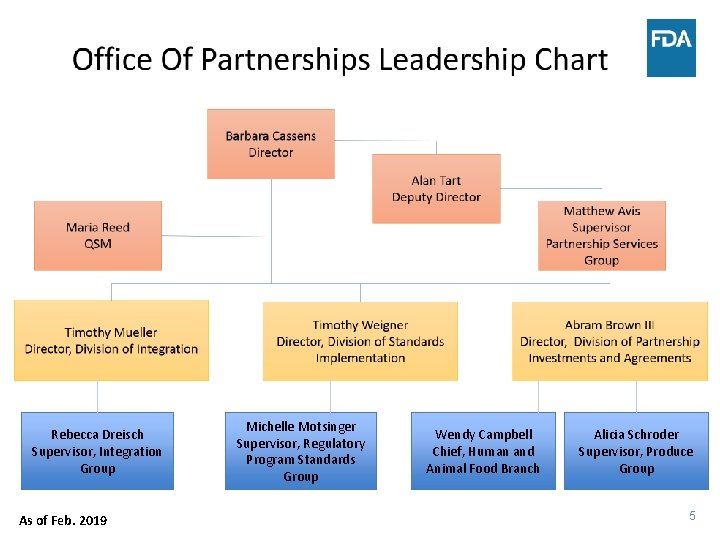
Rebecca Dreisch Supervisor, Integration Group As of Feb. 2019 Michelle Motsinger Supervisor, Regulatory Program Standards Group Wendy Campbell Chief, Human and Animal Food Branch Alicia Schroder Supervisor, Produce Group 5

DPIA: Division of Partnership Investments and Agreements • Develops, implements, monitors and evaluates select ORA contracts, grants, cooperative agreements (CAPs) and partnership agreements • Focus is human and animal food, eggs, medical products, & regulatory science • Coordinates outreach to SLTTs, associations, & organizations • Collaborates across FDA to ensure cohesive administration of funding agreements 6

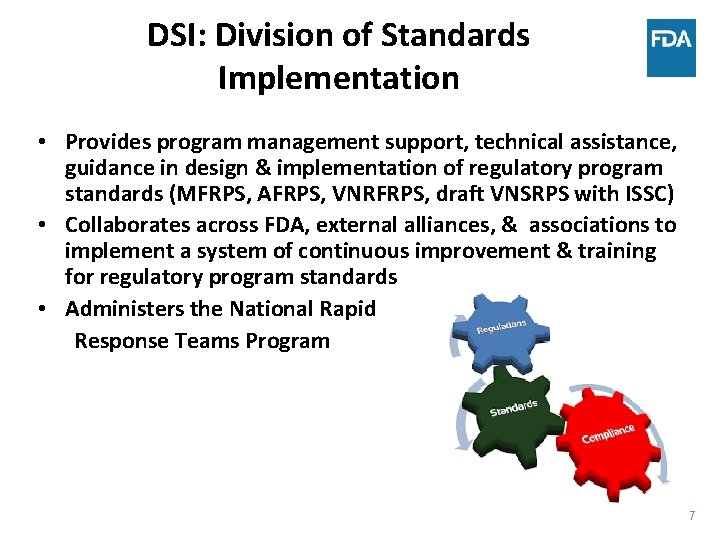
DSI: Division of Standards Implementation • Provides program management support, technical assistance, guidance in design & implementation of regulatory program standards (MFRPS, AFRPS, VNRFRPS, draft VNSRPS with ISSC) • Collaborates across FDA, external alliances, & associations to implement a system of continuous improvement & training for regulatory program standards • Administers the National Rapid Response Teams Program 7

DSI: Cooperative Programs • Provides leadership, technical assistance, consultation, guidance, and education with regulatory cooperative programs • Stakeholders: Federal, SLTT regulatory and public health agency officials, regulatory associations, consumers, academia, and industry Programs Include: • Retail Food • Shellfish Sanitation • Grade “A” Milk • Key players • OP • Office of State Cooperative Programs (OSCP) • Office of Training and Education Development • Center for Food Safety & Applied Nutrition 8

DI: Division of Integration • Reviews an array of processes to implement & promote a national Integrated Food Safety System (IFSS) • Develops, evaluates, and reports on national integration using performance measures of OP programs & awards • Devises strategies for addressing conflicts or duplication related to integration of federal, state & local activities • Provides oversight for ORA’s international interests, establishing and maintaining lines of communication within ORA, other FDA Offices, other domestic and foreign agencies, embassies, and international organizations 9

Fostering Federal Funding Opportunities to Support SLTT Agencies 10

Funding Initiative Overview • Coordination of ORA’s non training-focused contracts, grants, CAPs, & partnership agreements with SLTTs and others • Benefits: Emergency response, lab capacity, data sharing • Funding provides an opportunity to develop or enhance state regulatory infrastructure for new and existing programs in various regulated commodities 11

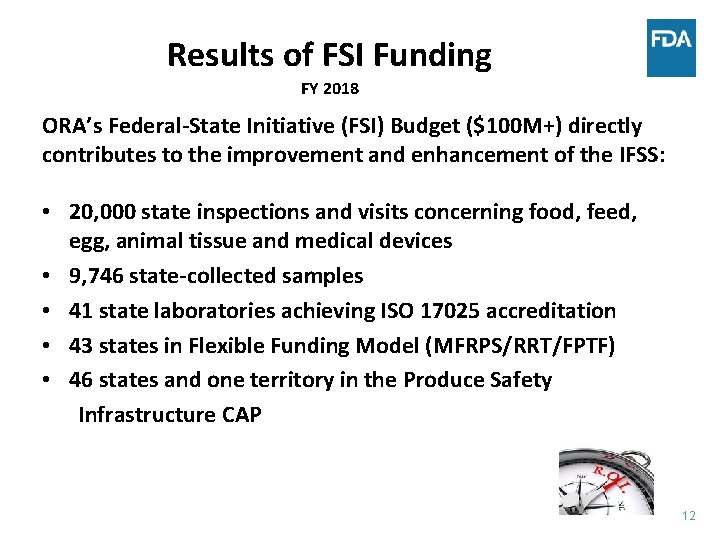
Results of FSI Funding FY 2018 ORA’s Federal-State Initiative (FSI) Budget ($100 M+) directly contributes to the improvement and enhancement of the IFSS: • 20, 000 state inspections and visits concerning food, feed, egg, animal tissue and medical devices • 9, 746 state-collected samples • 41 state laboratories achieving ISO 17025 accreditation • 43 states in Flexible Funding Model (MFRPS/RRT/FPTF) • 46 states and one territory in the Produce Safety Infrastructure CAP 12

ORA Inspectional Contracts Overview • 5 contract programs relate to the protection of public health – Human Food – Animal Food – Egg Safety – Medical Devices – Milk Database • FY 18 FSI Contract Funding – Human Food $12, 776, 842 – Animal Food $3, 384, 953 – Other Contracts $534, 223 OP administers, but does not fund, MQSA contracts 13

Egg Safety Contract Inspections Egg Safety • Currently contracted with 5 states in the inspectional coverage of egg layer farms to assess compliance with 21 CFR Part 118 14

Human Food Contract Program • States perform ~60% of the total human food inspections ORA coordinates • 48 state & territorial government agencies hold contracts • In 2018, this program contracted the work of: • 8, 334 Inspections • 7, 986 Sample collections • 305 Audits • 1, 253 Site visits* *Firm found to be Out of Business (OOB), not operational (inspection cannot be performed), not subject to FDA jurisdiction, or have moved more than 50 miles 15

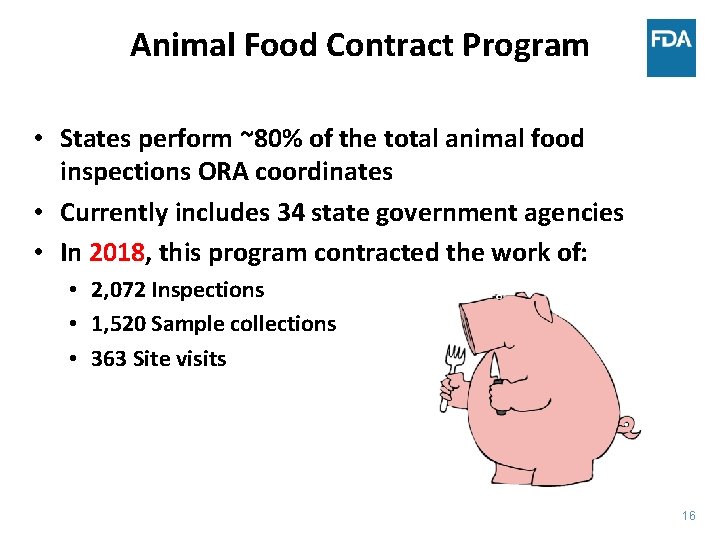
Animal Food Contract Program • States perform ~80% of the total animal food inspections ORA coordinates • Currently includes 34 state government agencies • In 2018, this program contracted the work of: • 2, 072 Inspections • 1, 520 Sample collections • 363 Site visits 16

Other FSI Contractual Programs Milk Database • National database that archives milk sample testing results for drug residues submitted by the industry and states Medical Device Inspection • Currently contracted with TX and CA to obtain state assistance in the inspection of Class I and Class II medical device manufacturers 17

MQSA Contract Program • Administers 42 contracts with state and territorial government agencies in inspectional coverage of non-federal, certified mammography facilities 18

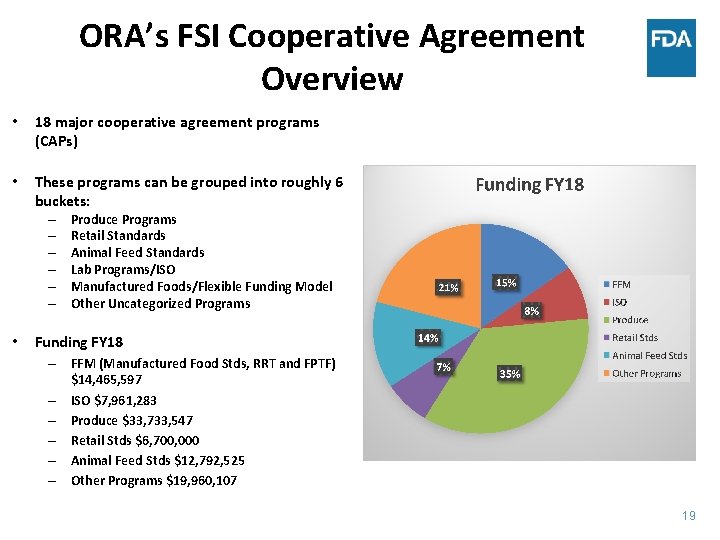
ORA’s FSI Cooperative Agreement Overview • 18 major cooperative agreement programs (CAPs) • These programs can be grouped into roughly 6 buckets: – – – • Produce Programs Retail Standards Animal Feed Standards Lab Programs/ISO Manufactured Foods/Flexible Funding Model Other Uncategorized Programs Funding FY 18 – FFM (Manufactured Food Stds, RRT and FPTF) $14, 465, 597 – ISO $7, 961, 283 – Produce $33, 733, 547 – Retail Stds $6, 700, 000 – Animal Feed Stds $12, 792, 525 – Other Programs $19, 960, 107 19

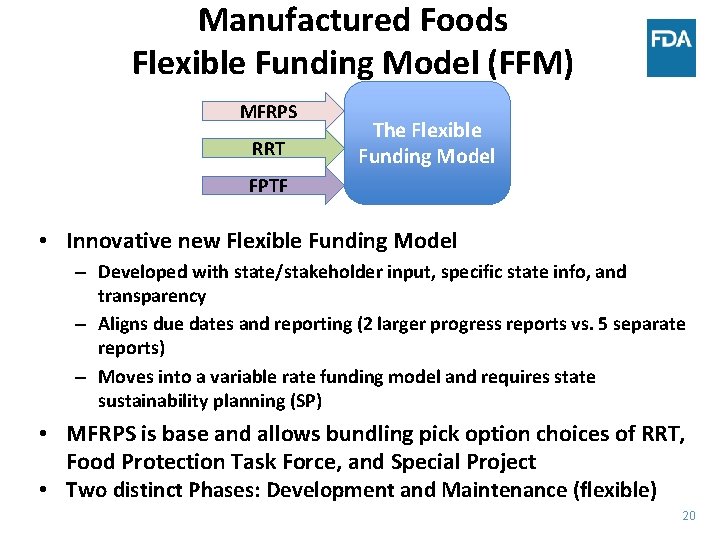
Manufactured Foods Flexible Funding Model (FFM) MFRPS RRT The Flexible Funding Model FPTF • Innovative new Flexible Funding Model – Developed with state/stakeholder input, specific state info, and transparency – Aligns due dates and reporting (2 larger progress reports vs. 5 separate reports) – Moves into a variable rate funding model and requires state sustainability planning (SP) • MFRPS is base and allows bundling pick option choices of RRT, Food Protection Task Force, and Special Project • Two distinct Phases: Development and Maintenance (flexible) 20

Supporting Produce Safety Rule (21 CFR 112) FDA’s Largest-Ever Cooperative Agreement • State Government Program – Assists states in creating a produce infrastructure and/or inspection program • Consensus Building Program – Developing a National Consortium for Produce Safety Development – On-Farm Advisory Coordination, Training and Review 21

Laboratory Funding Programs (Supporting FSMA Section 202) Food Emergency Response Network (FERN) & International Organization for Standardization (ISO) Accreditation Program 22

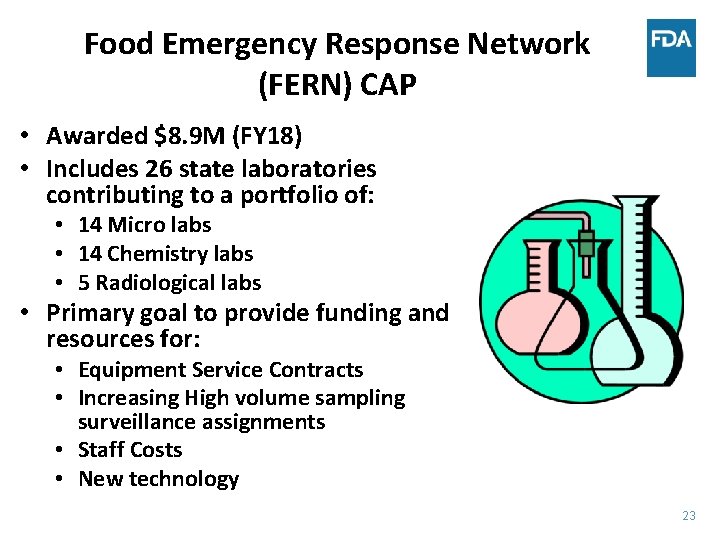
Food Emergency Response Network (FERN) CAP • Awarded $8. 9 M (FY 18) • Includes 26 state laboratories contributing to a portfolio of: • 14 Micro labs • 14 Chemistry labs • 5 Radiological labs • Primary goal to provide funding and resources for: • Equipment Service Contracts • Increasing High volume sampling surveillance assignments • Staff Costs • New technology 23

ISO and Laboratory Association Cooperative Agreement Programs (CAPs) • ISO 17025 Maintenance Goal: Assist labs to gain/maintain accreditation/expand methods • Whole Genome Sequencing (WGS) (New for FY 17) Goal: Perform basic foodborne pathogen identification during foodborne illness outbreaks and applying it in novel ways • $7. 9 M FY 18 Funding Total 24

Regulatory Program Standards • Supporting human, animal, and retail food safety through integration with national program standards • Bottom Line: Increasing the quality of State/Local regulatory program improves the overall consistency and confidence in the work conducted by these agencies 2017 25

Manufactured Food Regulatory Program Standards (MFRPS) • Purpose: Establishes a uniform foundation for the design and management of state manufactured food regulatory programs • Promotes quality regulatory programs through continuous self-improvements • Originally released in 2007, Updated September 2016 • Supports FSMA Section 201 and Integrated Food Safety Systems (IFSS) 26

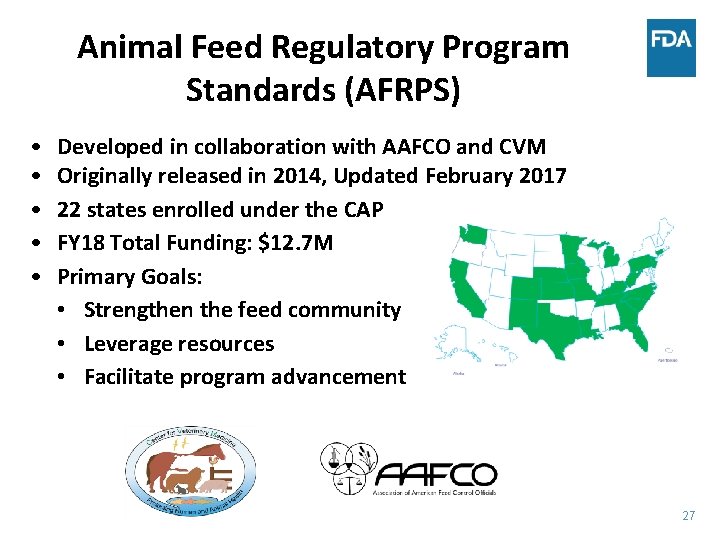
Animal Feed Regulatory Program Standards (AFRPS) • • • Developed in collaboration with AAFCO and CVM Originally released in 2014, Updated February 2017 22 states enrolled under the CAP FY 18 Total Funding: $12. 7 M Primary Goals: • Strengthen the feed community • Leverage resources • Facilitate program advancement 27

Voluntary National Retail Food Regulatory Program Standards (VNRFRPS) • Purpose: Guide to regulatory retail food programs managers in the design & management of a retail food program, and foundation for evaluation of effectiveness • 839 regulatory jurisdictions enrolled (as of 10/2/18) – State 61; Territory 5; County 488; District 77 (295 counties, 26 cities, 44 towns, 1 villages); City 117; Town 66; Tribe 11; Univ. 9; Federal Agency 4; Village 1. • 99. 74% - US population reside in a state with a retail food regulatory program standard enrollee • Total FY 18 Funding (OP): $6. 70 M* *Retail Association program administered by CFSAN, paid by OP 28

Rapid Response Teams (RRT) Programs Focuses on • Developing advanced response capacity (allhazards food/feed emergency response) RRT is a team effort • Multi-agency, multi-disciplinary Sharing what we’ve learned • RRT Best Practices Manual • Mentorship 29

Initiatives • Integration Assessment Model • Measures national progress towards an IFSS • Mutual Reliance Pilots • Designed to promote data sharing, work planning, and reliance on each other’s work products 30

Sharing Information with FDA Stakeholders: Commissioning, Credentialing and 20. 88 Agreements Purpose • Delegation of federal authority • Human and animal food, milk, produce, egg, tissue residue, BSE, drugs, devices, tobacco, rad health Current Numbers • ~3, 200 commissioned officials as of Feb. 2019 Integration – Long Term Goals • Reduce/Replace Certificates of Commissions in human food • 20. 88 Information Sharing Agreements 31

Partnership for Food Protection (PFP) • Leveraging resources, talents, expertise to build and achieve integrated food safety systems (IFSS) and public health outcomes Source: https: //www. pfp-ifss. org 32

Recap of Current Egg Standard Cooperative Agreement – Program Objectives • To develop recommendations for elements of a National Egg Regulatory Program Standard for state egg regulatory programs using the MFRPS and AFRPS as guidance • To develop information sharing techniques and systems between FDA and states • To encourage joint inspections between FDA and states • Participating Grantees – CA and IA 33

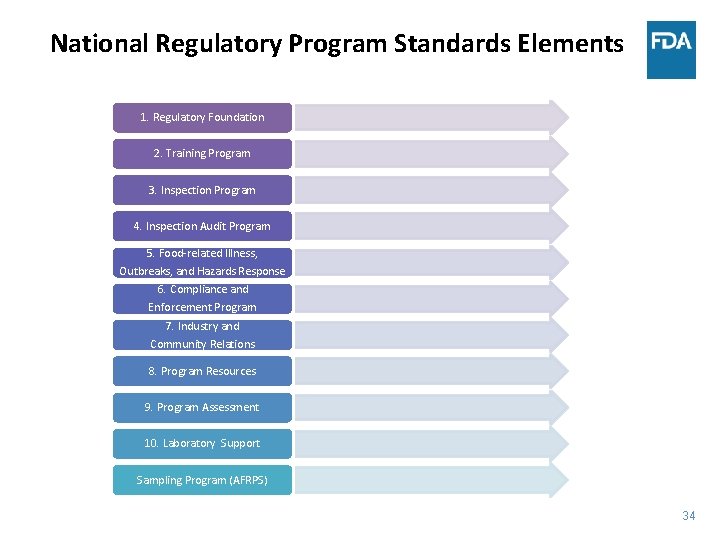
National Regulatory Program Standards Elements 1. Regulatory Foundation 2. Training Program 3. Inspection Program 4. Inspection Audit Program 5. Food-related Illness, Outbreaks, and Hazards Response 6. Compliance and Enforcement Program 7. Industry and Community Relations 8. Program Resources 9. Program Assessment 10. Laboratory Support Sampling Program (AFRPS) 34

Egg Standard Cooperative Agreement Program Next Steps • FDA will allow the current Egg CAP to end this summer and focus on development of standards • Collaborate with NERO to facilitate a more effective long-term approach to developing and maintaining standards – Leadership support moving forward – Build on lessons learned and recommendations from pilot states (CA and IA) – Engagement of necessary stakeholders (e. g. , NERO, states, FDA, others) – Determine process for development, consensus and timelines – Determine ownership of the standards – Develop communication and education strategies to support the standards Standard Development Workgroup Standards Development Phase Clearance Phase Roll-out & Implementation Phase Maintenance Phase 35

Egg Standard Cooperative Agreement Program Next Steps • Workgroup could immediately begin developed to create the process and standards – OP could fund travel for workgroup meetings, as necessary • Decision to develop a new FOA could be made in summer 2019 • Cooperative agreement funding could be made available as early as summer 2021. Dependent on – Annual appropriations – Participation estimates – number of participating states and their funding needs – Commitment and support of NERO members to assist in the development of the standards 36

Questions

Office of Partnerships 38

Feedback • OP welcomes your feedback on this presentation and any services provided by OP • Any feedback provided to this address will be reviewed by the Director of Office of Partnerships OP. Feedback@fda. hhs. gov 39

 Udai working together works
Udai working together works Working together works
Working together works Safety at street works and road works a code of practice
Safety at street works and road works a code of practice Hard work vs smart work
Hard work vs smart work Advantage of hot working process
Advantage of hot working process Hot working and cold working difference
Hot working and cold working difference Differentiate between hot working and cold working
Differentiate between hot working and cold working Proses pengerjaan panas
Proses pengerjaan panas Working together to safeguard children summary
Working together to safeguard children summary Teamwork animals working together
Teamwork animals working together Short poems about teamwork
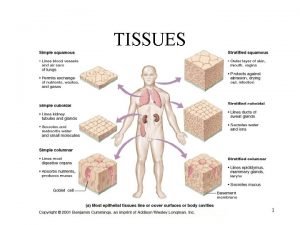
Short poems about teamwork A group of similar cells working together.
A group of similar cells working together. Tissues are groups of similar cells working together to
Tissues are groups of similar cells working together to Working together to achieve a common goal
Working together to achieve a common goal Root hair cells
Root hair cells Wtpn
Wtpn Unit 2 leadership and teamwork in the public services
Unit 2 leadership and teamwork in the public services Group cohesiveness and performance
Group cohesiveness and performance Tissues are groups of similar cells working together to
Tissues are groups of similar cells working together to Teachers and teacher aides working together
Teachers and teacher aides working together Teachers and teacher aides working together
Teachers and teacher aides working together Working together agreement
Working together agreement Working better together
Working better together Paraprofessionals and teachers working together
Paraprofessionals and teachers working together Words of wisdom about working together
Words of wisdom about working together Tissues working together
Tissues working together Churches and schools working together
Churches and schools working together Teachers and teacher aides working together
Teachers and teacher aides working together Working together for change
Working together for change Volunteers working together
Volunteers working together Working better together
Working better together Group of cells working together
Group of cells working together A group of cells similar in structure and function
A group of cells similar in structure and function Organs working together
Organs working together Marco koper
Marco koper Accounting for partnerships chapter 12 solutions
Accounting for partnerships chapter 12 solutions Accenture international development
Accenture international development Marketing word partnerships
Marketing word partnerships Product development partnerships
Product development partnerships Characteristics of just-in-time partnerships do not include
Characteristics of just-in-time partnerships do not include Maintaining effective partnerships
Maintaining effective partnerships