Office for Clinical Research OCR Consistency in OCR










- Slides: 20

Office for Clinical Research (OCR) Consistency in OCR Budgeting Based on Historical Results Karen Manion Sally Mountcastle 1

Background • Currently the OCR is divided into two teams. Team 2 is responsible for Winship Cancer Institute and Radiation Oncology clinical trials. Team 1 is responsible for studies not involving cancer. • The OCR develops and negotiates budgets for all clinical trials conducted at Emory that have billable CPT Code driven items/services for a multitude of Industry Sponsors.

Continued Background • Each clinical trial is assigned to a Clinical Research Finance Manager (CRFM) for budget development and negotiations. • Documented guidance is utilized regarding administrative/site costs, i. e. Start-up Fees, Monitoring Fees and Record Retention. • There is variability among CRFMs as to initial budget offer to the sponsor for specific fees.

Continued Background • Currently the process to determine the amount paid historically by a sponsor for a particular item/procedure, is to go through each study file and search for the final budget. • To maintain our internal productivity goal of budget negotiation completion within 25 days, efficiency is important.

Continued Background • It is time consuming to search past study records for the last negotiated budget- for example, our top sponsor represents approximately 20 budgets to review. • We have chosen our five top sponsors (by number of studies) and eight items/procedures that may vary in amount negotiated by the CRFM and by the individual sponsor.

AIM STATEMENT To create a sponsor specific template of commonly negotiated items/procedures in clinical trials to decrease the variability in negotiated costs by each CRFM. Our goal is to have 100% compliance in the use of this tool by all CRFMs for the top five sponsors beginning with budget development as of June 1, 2014.

WHERE DO WE START? ?

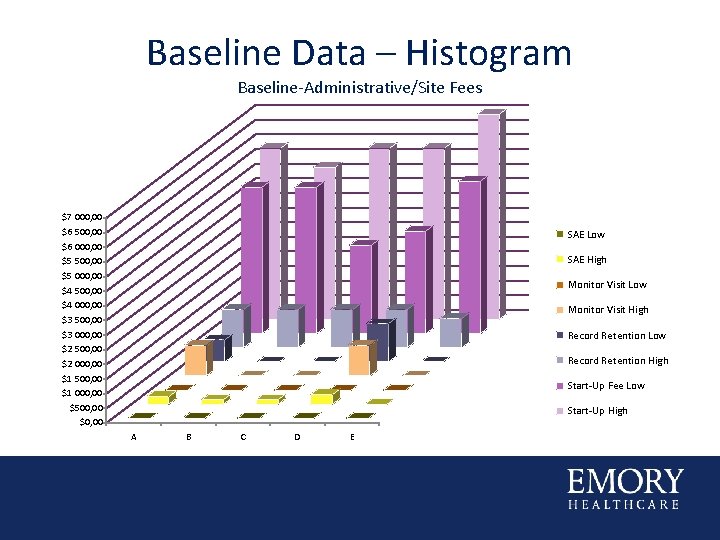
Baseline Data – Histogram Baseline-Administrative/Site Fees $7 000, 00 $6 500, 00 $6 000, 00 $5 500, 00 $5 000, 00 $4 500, 00 $4 000, 00 $3 500, 00 $3 000, 00 $2 500, 00 $2 000, 00 $1 500, 00 $1 000, 00 $500, 00 $0, 00 SAE Low SAE High Monitor Visit Low Monitor Visit High Record Retention Low Record Retention High Start-Up Fee Low Start-Up High A B C D E

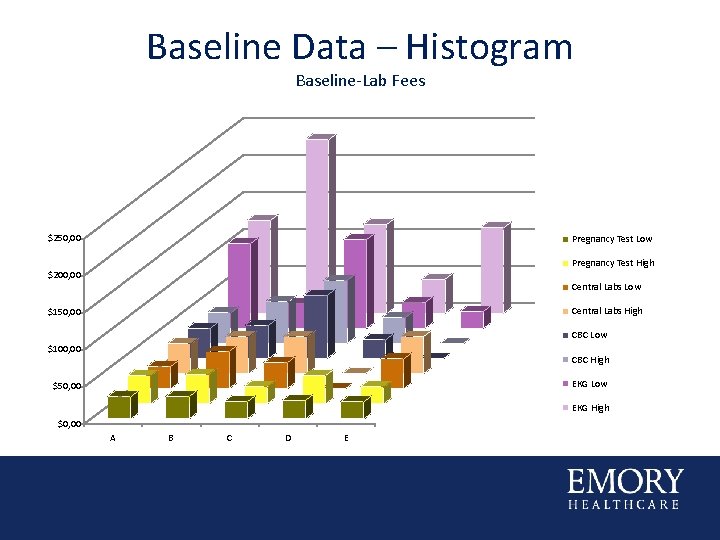
Baseline Data – Histogram Baseline-Lab Fees $250, 00 Pregnancy Test Low Pregnancy Test High $200, 00 Central Labs Low Central Labs High $150, 00 CBC Low $100, 00 CBC High EKG Low $50, 00 EKG High $0, 00 A B C D E

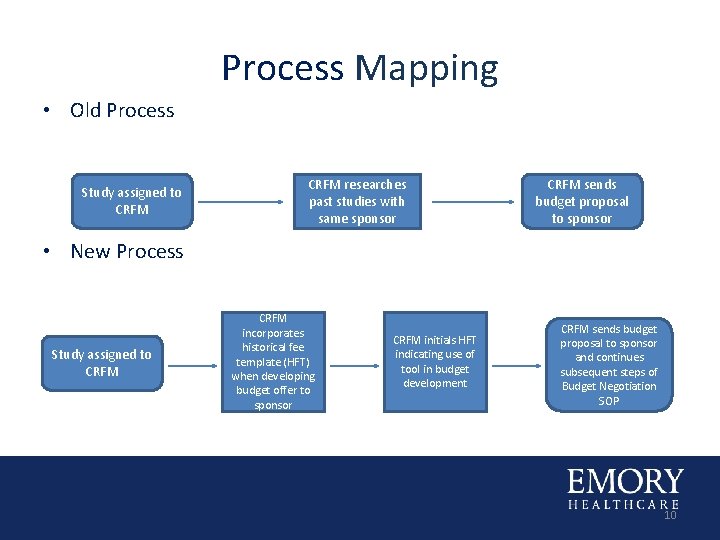
Process Mapping • Old Process Study assigned to CRFM researches past studies with same sponsor CRFM sends budget proposal to sponsor • New Process Study assigned to CRFM incorporates historical fee template (HFT) when developing budget offer to sponsor CRFM initials HFT indicating use of tool in budget development CRFM sends budget proposal to sponsor and continues subsequent steps of Budget Negotiation SOP 10

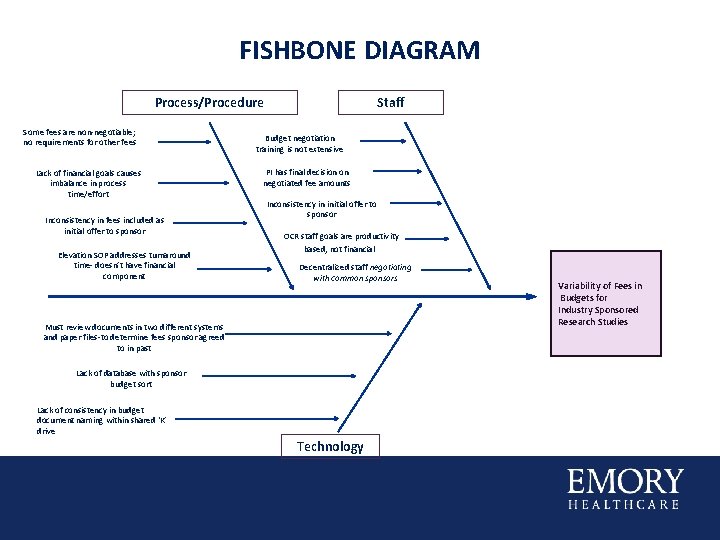
FISHBONE DIAGRAM Process/Procedure Some fees are non-negotiable; no requirements for other fees Lack of financial goals causes imbalance in process time/effort Inconsistency in fees included as initial offer to sponsor Elevation SOP addresses turnaround time- doesn’t have financial component Staff Budget negotiation training is not extensive PI has final decision on negotiated fee amounts Inconsistency in initial offer to sponsor OCR staff goals are productivity based, not financial Decentralized staff negotiating with common sponsors Must review documents in two different systems and paper files-to determine fees sponsor agreed to in past Lack of database with sponsor budget sort Lack of consistency in budget document naming within shared ‘K’ drive Technology Variability of Fees in Budgets for Industry Sponsored Research Studies


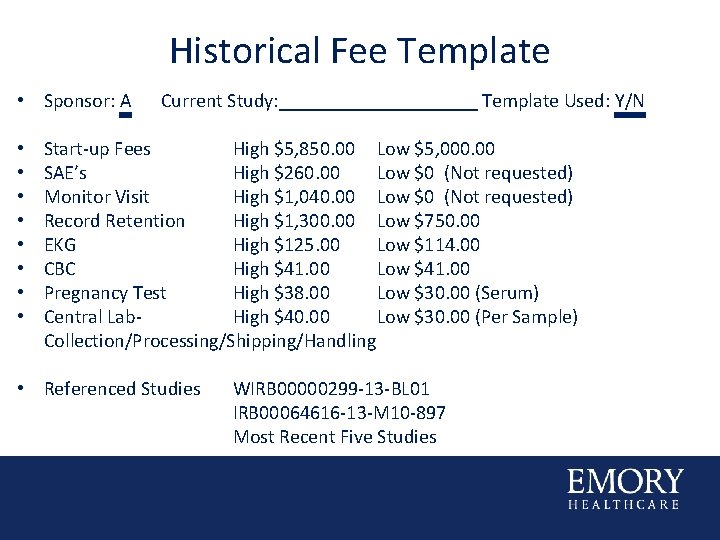
Historical Fee Template • Sponsor: A • • Current Study: __________ Template Used: Y/N Start-up Fees High $5, 850. 00 Low $5, 000. 00 SAE’s High $260. 00 Low $0 (Not requested) Monitor Visit High $1, 040. 00 Low $0 (Not requested) Record Retention High $1, 300. 00 Low $750. 00 EKG High $125. 00 Low $114. 00 CBC High $41. 00 Low $41. 00 Pregnancy Test High $38. 00 Low $30. 00 (Serum) Central Lab. High $40. 00 Low $30. 00 (Per Sample) Collection/Processing/Shipping/Handling • Referenced Studies WIRB 00000299 -13 -BL 01 IRB 00064616 -13 -M 10 -897 Most Recent Five Studies

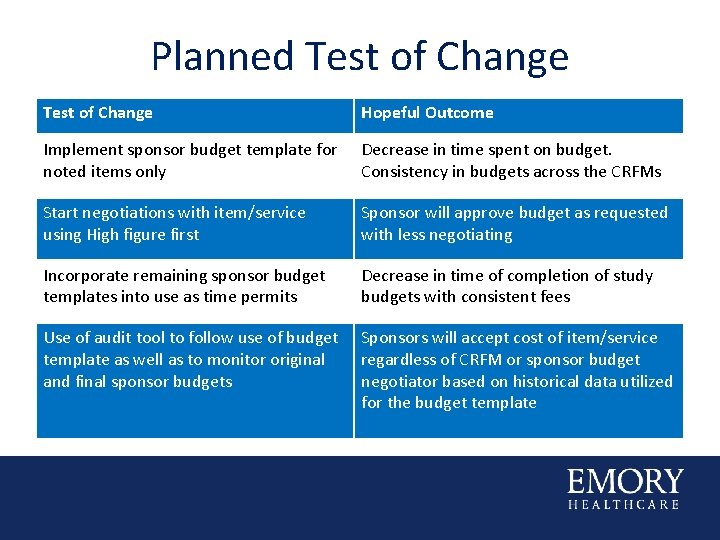
Planned Test of Change Hopeful Outcome Implement sponsor budget template for noted items only Decrease in time spent on budget. Consistency in budgets across the CRFMs Start negotiations with item/service using High figure first Sponsor will approve budget as requested with less negotiating Incorporate remaining sponsor budget templates into use as time permits Decrease in time of completion of study budgets with consistent fees Use of audit tool to follow use of budget template as well as to monitor original and final sponsor budgets Sponsors will accept cost of item/service regardless of CRFM or sponsor budget negotiator based on historical data utilized for the budget template

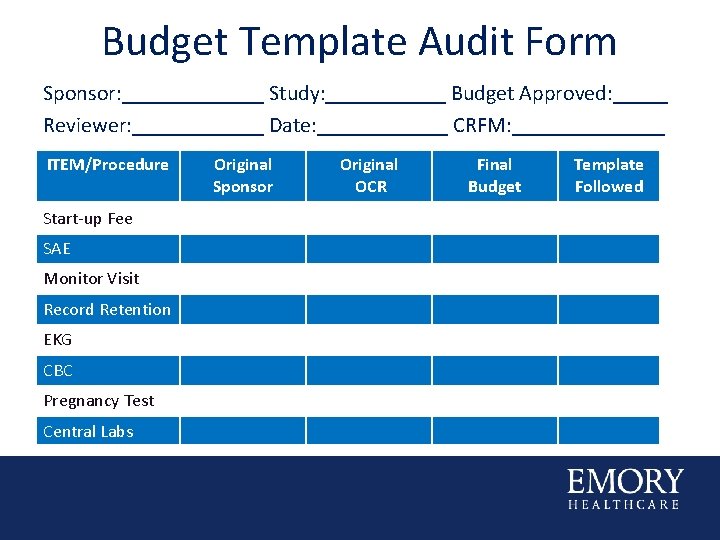
Budget Template Audit Form Sponsor: _______ Study: ______ Budget Approved: _____ Reviewer: ______ Date: ______ CRFM: _______ ITEM/Procedure Start-up Fee SAE Monitor Visit Record Retention EKG CBC Pregnancy Test Central Labs Original Sponsor Original OCR Final Budget Template Followed

Barriers? ?

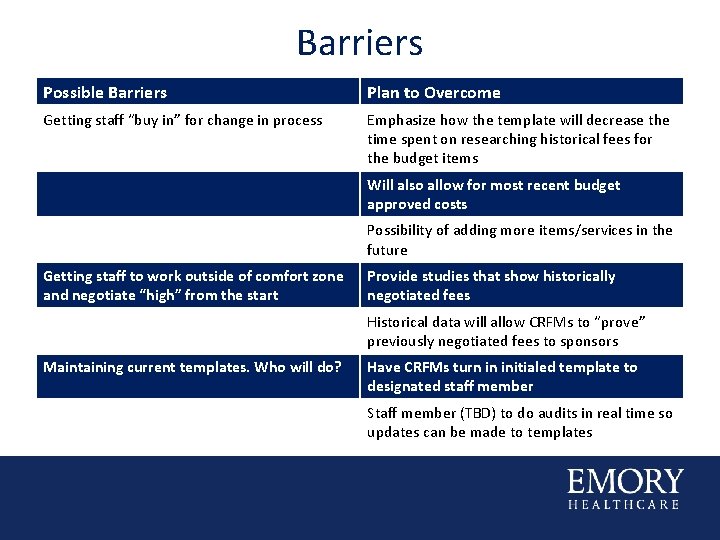
Barriers Possible Barriers Plan to Overcome Getting staff “buy in” for change in process Emphasize how the template will decrease the time spent on researching historical fees for the budget items Will also allow for most recent budget approved costs Possibility of adding more items/services in the future Getting staff to work outside of comfort zone and negotiate “high” from the start Provide studies that show historically negotiated fees Historical data will allow CRFMs to “prove” previously negotiated fees to sponsors Maintaining current templates. Who will do? Have CRFMs turn in initialed template to designated staff member Staff member (TBD) to do audits in real time so updates can be made to templates

Next Steps • In-service staff on use of sponsor budget template. • Emphasize the need for consistency in budget preparation as well as the decrease in time spent research last approved sponsor budget. • Audit the use of the individual sponsor templates. • Continue to implement budget templates for the remaining sponsors.

Terry Anderson

Questions? ?