NUTRIENT INTERACTION Nutrient A chemical compound such as













- Slides: 40

NUTRIENT INTERACTION

Nutrient A chemical compound (such as protein, fat, carbohydrate, vitamins, or minerals) that make up foods. These compounds are used by the body to function and grow. Nutrient can be classified as Macronutrients There are three macronutrients defined as being the classes of chemical compounds humans consume in the largest quantities and which provide bulk energy. These are organic nutrients like PROTEIN, FAT and CARBOHYDRATES. Micronutrients These are inorganic nutrients such as minerals and vitamins which are required by body in very small quantities. Nutrient Interaction It can be defined as the physical chemical interaction between nutrients, or between nutrients and other components of the diet or other compounds, including desirable or undesirable results.

Carbohydrate –carbohydrate interaction Carbohydrates are important nutrient which provide energy to our body. It is an organic compound made up by carbon , hydrogen and oxygen. 1. Inter- relation between Fructose and Sucrose When fructose is ingested as a part of the dissaccharide sucrose , absorption capacity is much higher because fructose exists in a 1 : 1 ratio with glucose. In addition , serum galactose levels following galactose ingestion are reduced when accompanied by glucose. 2. Inter –relation between Carbohydrate – Fiber FIBER is a type of polysaccharides which found in plants and it gives structure to plants. There is a 2 type of fibers like soluble and insoluble fiber. Soluble fiber such as pectin etc mixes with water to form gummy substances that coats the insides of the intestinal tract

During digestion, wave-like currents caused by contractions of the intestinal muscles bring nutrients to the surface of the intestinal wall for absorption. After soluble fiber dissolves in water, however, it traps nutrients inside its gummy gel and slows down considerably while moving through the digestive tract. Inside the gel, nutrients are shielded from digestive enzymes and less likely to reach the wall of the intestines. Dietary sugars like carbohydrates and starch are among the nutrients trapped inside this gel. Consequently, sugar is absorbed into the bloodstream more slowly, blunting the sharp spike in blood glucose typically experienced by diabetic patients after a meal. Fewer spikes in blood glucose lead to greater sensitivity to the action of insulin. Avoiding high peaks and low valleys in blood glucose places less stress on the pancreas and is important not only to diabetics but also to those who want to prevent the development of type 2 diabetes

Glycemic Index The glycemic index is a measure of the effects of carbohydrates in food on blood sugar levels. It estimates how much each gram of available carbohydrate (total carbohydrate minus fiber) in a food raises a person's blood glucose level following consumption of the food, relative to consumption of glucose. Plasma glucose level rise 5 -45 min after any meal that contains sugars or digestible starch and return to fasting levels 2 -hours later. White bread has a glycemic index of 100 and other foods have a lower glycemic index. Foods with a high glycemic index, such as processed starches and the sugar in soft drinks, break down into glucose and enter the bloodstream relatively quickly. Unrefined, complex carbohydrates, on the other hand, have a low glycemic index and digest more slowly. Diabetic patients should consume food with a low glycemic index because rapid increases in blood glucose exacerbate overproduction of insulin by the pancreas and insulin resistance.

The glycemic index depends on: 1. Composition and size of starch particles Smaller the particle size more is the glycemic effect. Raw foods with large particles therefore have a lower effect on glycemic index. 2. Their digestibility Presence of amylopectin that gets rapidly digested also has a greater glycemic effect whereas the amount of amylose which is digested slowly has low gycemic index. 3. Cooking methods employed Foods cooked by boiling and long cooking process makes it easy to digest and reduces the particle size thus increasing the glycemic index.

Carbohydrate –Protein Inter-relations 1. Carbohydrate and Hormones § Glucagon, a hormone secreted by the pancreas, raises blood glucose levels. Its effect is opposite that of insulin, which lowers blood glucose levels. The pancreas releases glucagon when blood sugar (glucose) levels fall too low. Glucagon causes the liver to convert stored glycogen into glucose, which is released into the bloodstream and also from non CHO substances like amino acids etc. High blood glucose levels stimulate the release of insulin. Insulin allows glucose to be taken up and used by insulin-dependent tissues. Thus, glucagon and insulin are part of a feedback system that keeps blood glucose levels at a stable level. So, Glucagon is responsible for gluconeogenesis and glycogenolysis.

§ Cortisol It is a hormone produced by adrenal gland. Its function is to increase blood sugar through gluconeogenesis ; suppress the immune system; and aid in fat, protein and carbohydrate metabolism so , it is an overall catabolic hormone , which decreases lean body mass and muscle mass and may increase energy expenditure. Cortisol withdrawal increase insulin sensitivity interms of increased glucose oxidation and decrease glucose production. This may include hypoglycemia in adrenocortical failure. 2. Protein sparing action During fasting or starvation or insufficient carbohydrates and fats for fuel , body stores of glycogen are exhausted. Body adapts to use of muscle protein to meet most of the need of glucose production , mainly needed for brain , RBCs etc, and this is done by gluconeogenesis. But to use protein instead of carbohydrates to give energy is not a wise contribution as the urinary nitrogen excretion increases during starvation. If carbohydreates are sufficient, then protein can be spared of tissue building process.

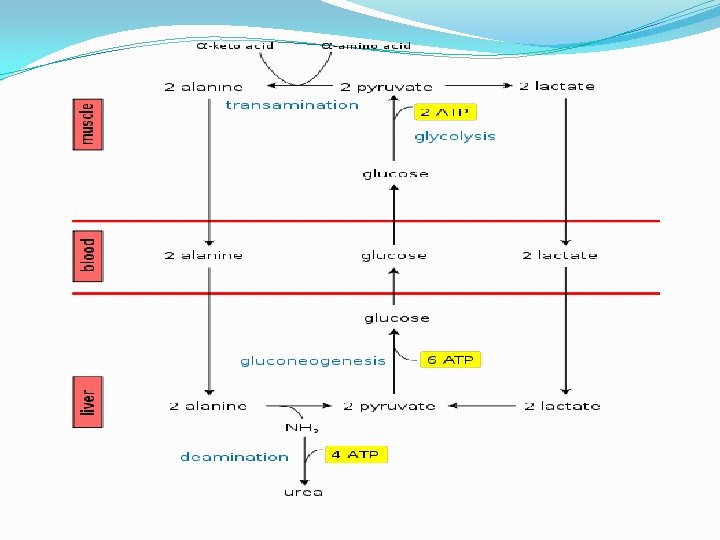
3. Glucose and Alanine �Alanine plays a key role in glucose–alanine cycle between tissues and liver. In muscle and other tissues that degrade amino acids for fuel, amino groups are collected in the form of glutamate by transamination. Glutamate can then transfer its amino group through the action of alanine aminotransferase to pyruvate forming alanine and α-ketoglutarate. The alanine formed is passed into the blood and transported to the liver. � A reverse of the alanine aminotransferase reaction takes place in liver. Pyruvate regenerated forms glucose through gluconeogenesis, which returns to muscle through the circulation system. Glutamate in the liver enters mitochondria and degrades into ammonium ion through the action of glutamate dehydrogenase, which in turn participate in the urea cycle to form urea. �The glucose–alanine cycle enables pyruvate and glutamate to be removed from the muscle and find their way to the liver. Glucose is regenerated from pyruvate and then returned to muscle: the energetic burden of gluconeogenesis is thus imposed on the liver instead of the muscle. All available ATP in muscle is devoted to muscle contraction


4. Fiber and Trypsin Fiber i. e. cellulose has also been shown to reduce the actvitiy of human pancreatic trypsin in protein digestion. This is shown as slightly increased faecal loses of nitrogen on increased fiber diet. In addition amylase and lipase activity is also depressed. 5. Maillard reaction When a reducing sugar is heated with protein , a maillard reaction occurs that reduces the availability of some amino acids , like lysine. The monosaccharide in intestinal lumen may influence rate of uptake of certain amino acids , fructose seeming to accelerate this reaction e. g. During milk processing or heat treatment , milk sugar lactose react with free side chains of lysine residues to render it unavailable. Under sever heating conditions, in presence of sugar , food protein becomes resistant to digestion so that availability of amino acid is reduced.

6. Enzymes and carbohydrate hydrolysis When enzymes concerned with hydrolysis of carbohydrate are missing or inadequate, common symptom is osmotic diarrhea. This condition may arise because of congenital absence of appropriate enzyme required for digestion of lactose , sucrose or maltose. Inadequancies of these enzyme may also be secondary to gut mucosal damage due to such condition as celiac disoder or protein deficiency. 7. Glucose and tryptophan Amino acid uptake across the blood brain barrrier is influenced indirectly by serum glucose in that the insulin concentration is directly related to movement of tryptophan into brain. The disorders fructose malabsorption and lactose intolerance cause improper absorption of tryptophan in the intestine, reduced levels of tryptophan in the blood and depression. 8. Glucosamine and Collagen Glucosamine (an amino monosaccharide found in chitin , glycoproteins and glycosaminoglycans such as hyaluronic acid and heparin sulfate) provides the primary substrate for both collagen and proteoglycan synthesis.

9. Genetic errors may also occur in conversion of fructose and galactose. Absence of enzyme fructokinase in liver prevents fructose breakdown and is excreted in urine i. e. fructosuria. Diminished activity of fructose -1 -phosphate aldose in liver results in hypoglycemia and hypophosphatemia with associated vomiting. Clinical forms of glactosemia occur as inborn errors of metabolism and result of enzyme deficincies. Deficient enzyme is galactokinase in which galactose is not phosphorylated. This may lead to cataract in otherwise normal subject. Also glucose-6 -phosphate dehdrogenase deficiency result in inability to maintain glutathione in reduced form during exposure to drugs such as sulfonamides or some antimalarials , leading to haemolysis or anemia.

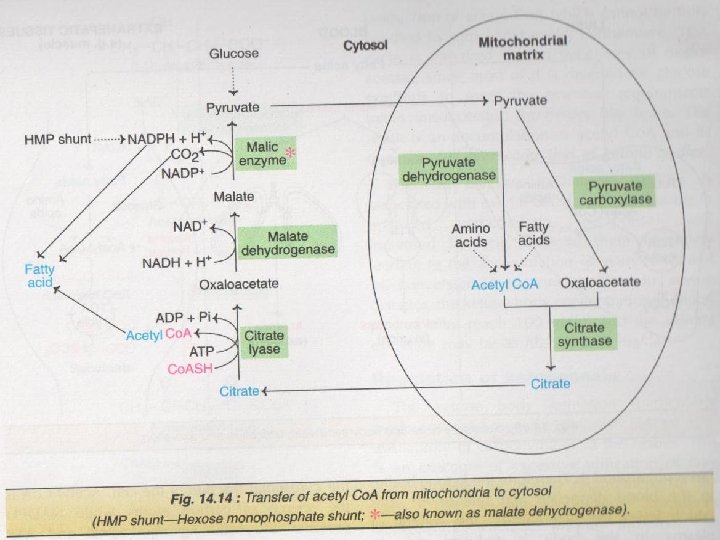
Carbohydrate – Fat Inter-relation 1. Conversion into fat Glucose is a six-carbon sugar molecule and body first converts this molecule into two three-carbon pyruvate molecules through the process of glycolysis and then into acetyl Co. A. When body requires immediate energy, acetyl Co. A enters the Citric Acid Cycle creating energy molecules in the form of ATP. But when glucose intake exceeds then acetyl Co. A begins the process of fatty acid synthesis becoming triglycerides that are stored in the fat tissues of body. These triglycerides are stored energy molecules which can be broken down later to give energy when need , for example, get up off the couch and go for a bike ride. � Regulation of Fatty Acid Synthesis Fatty acid synthesis is influenced by foods which we eat and hormones we release. When blood glucose levels are high, such as after eating a sugary meal, body releases insulin. Insulin stimulates the formation of Fatty Acid Synthase, an enzyme that increases fat storage.

� On the other hand, polyunsaturated fatty acids decrease the formation of the Fatty Acid Synthase enzyme, implying that eating foods containing polyunsaturated fats may not lead to as much increased fat storage as eating sugary foods. In addition, when fat cells increase their fat storage, a molecule called leptin is produced. Leptin leads to decreased food intake, increased energy expenditure, as well as inhibition of fatty acid synthesis.


2. Prevent ketosis The primary function of CHO is to provide energy. However during low CHO intake , fats are mobilized to meet the energy requirement of body. This result in increased plasma free fatty acids and ketone bodies. Hence, sufficient amount of CHO spares fats from being broken down. 3. Chitin and Cholesterol Chitin (a polysaccharide found in the exoskeleton of some invertebrates e. g. Insects , crustaceans 0 and chitosan, have hypocholestrolemic effect. The strong positive charge on chitosan binds negatively charged lipids blocking their absorption. 4. Fiber and Lipid Serum lipid concentration can be modified by insoluble fibers such as cellulose , lignin, chitin and more soluble fibers because v Fibers bind faecal bile acid and increases excretion of bile acidderived cholesterol. v Fiber prevents dietary fat and cholesterol absorption by binding bile acids or fats and lipids.

v Fermentable oligosaccharides and dietary fiber are converted by intestinal bacteria to short chain fatty acids, which lower blood lipids by mechanisms that are currently unclear. So Fiber decreases the absorption of dietary cholesterol from the intestine. Further , fiber binds with bile salts and reduces their enterohepatic circulation. This cause increased degradation of cholesterol to bile salts and its disposal from the body. Carbohydrate-Mineral interrelations Carbohydrate and Zinc Highe fiber diet is associated with zinc deficiency. Zinc absorption may be enhanced by glucose and lactose intake.

Carbohydrate and Calcium It is seen that lactose improves the absorption of calcium from the gut. Even in adults with lactose intolerance , lactose probably improves Ca absorption. Sugars and organic acids produced by microbial fermentation of sugars in the gut increases the solubility of calcium salts and increases their absorption. Fiber may decrease calcium absorption , this process occurs if calcium intake is more than 30 gm per day. 3. Carbohydrate and Iron Wheat bran includes low serum iron levels as they contains phytate which inhibits iron absorption. In this regard iron absorption from unpolished rice is significantly worse then from polished rice.

4. Phosphorus and Carbohydrates Phosphorus plays an essential part in carbohydrate metabolism in phoshoryalation of glycogen. Phosphorus is an essential constituent of coenzyme I and co- carboxylase enzyme system in the oxidation of carbohydrate , fat and protein. . 5. Copper and Fiber Dietary fiber do not inhibits copper absorption. 6. Chromium and Carbohydrates The high sugar diet enhanced urinary chromium losses 7. Magnesium and carbohydrates Magnesium deficiency has been linked to insulin resistance and metabolic syndrome because magnisium is required for CHO metabolism. Increased intakes of dietary fiber have been reported to decrease magnesium utilization in humans presumably by decreasing absorption.

Vitamin- Carbohydrates inter-relations 1. Vitamin –C and Carbohydrate Vitamin – C has been found to affect the regulating CHO metabolism either at the level of rate of absorption of CHO from intestine , or of glycogen level alteration of liver and other tissues. It has been found that in scorbutic animals , there is a diminution of glutathione levels with simultaneously depression in insulin secretion. This is due to the reason as there is an increase in dehydro ascorbic level in tissues which may combine with sulfydral groups of glutathione making it unavailable for the protective role in beta cells of pancreas and causes diminished insulin secretion.

There is a severe depression in hexokinase activity and in the turnover rate of phosphorylated intermediates of CHO metabolism in scorbutic conditions. A depression in phosphoglucomutase and phosphohexoseisomerase activity with stimulation in glucose -6 -phosphate dehydrogenase activity were noted. The depression in glycogen synthesis in scurvy was mostly due to the limiting availability of uridine triphosphate and the diminished activities of hexokinase and phosphoglucomutase under vitamin-C deprivation. In scorbutic conditions, there is also a depression in the TCA cycle and electron transport chain whee V-C act as electron acceptor and its function is highly specific.

2. Biotin and Carbohydrate Replacing glucose in the diet with other carbohydrates of low molecular weight like sorbitol and fructose elevates the severity of biotin deficiency. Since glucose utilization is impaired , it is likely that provision of other CHO, improves the energy supply.

LIPID-LIPID INTER-RELATIONSHIPS Lipids may be regarded as organic substances relatively insoluble in water , soluble in organic solvents (alcohol, ether etc ) , actually or potentially related to fatty acids and utilized by the living cells. Lipids are the concentrated form of energy. Lipid lipid interaction is that in which TRANS FATTY acids inhibit the desaturation and elongation of linoleic acid and alpha –linolenic acid to form long chain essential fatty acids. Trans fatty acid -----Linoleic acid ----- alpha – linolenic acid--Essential fatty acid Trans fatty acids The food industry incorporates fats and oils into margarines , biscuits, cake, chocolates and other manufactures products. Food manufactures use fats and oils that have been

�altered by the process of hydrogenation, i. e. adding hydrogen atoms to the double bonds in monounsaturated fatty acids and polyunsaturated fatty acids in order to increase the degree of saturation of fatty acids in the oils. Hydrogenation changes the configuration of some monounsaturated fatty acids and polyunsaturated fatty acids. Cis fatty acids have two hydrogen atoms attached to the carbon on the same side of the double bond and molecule bends at the double bond. In trans fatty acids , the hydrogen atoms are placed on the opposite sides of the double bond and the molecule stays straight at the double bond. Trans fatty acids behave biologically as saturated fats rather than like cis unsaturated fatty acids. The bulk of trans fatty acids in hydrogenated fats are monounsaturated fatty acid – Eladic acid which is trans equivalent to oleic acid.

Most of the dietary intake of fatty acids is derived from margarine , dalda and other foods manufactures from hydrogenated fats. Saturated and short chain fatty acids Stearic acid is a saturated fatty acid with no double bond. It lowers the HDL but does not raise serum cholesterol reducing both total and saturated fat. Short chain fatty acids are organic anoins predominantly acetate , butyrate and propionate. In the caecum , these exist in the production of 70%, 20% and 10% respectively. Short chain fatty acids are produced by the colonic bacteria from unabsorbed carbohygrates. They are utilized as a source of energy by large intestine and stimulate its mucosal growth. The fatty acids hydrolyzed from short chain fatty acids are transported to the liver as free acids via the portal vein. They enter the mitochondria of the liver cells and are oxidised rapidly. Short chain fatty acids Large intestine Carbohydrates

Protein- Lipids inter- relationships 1. Starvation Conditions If gluconeogenesis were to contiue at accelerated rate during early starvation , skeletal muscles would soon be exhausted. An adaptation in lipid metabolism occurs in long term starvation so that ketone bodies (acetoacetate, beta hydroxybutyrate) are formed. Ketone bodies cross blood barrier to provide energy to brain and thereby spare body protein from degradation. Production and utilization of these ketone bodies result in reduction in protein degradation and oxidation of amino acids. These adaptations help conserve both energy and amino acids and is reflected in output of nitrogen in urine , which is decreased from 12 gms in early starvation to 3 gms nitrogen per day by several weeks of starvation.

�When body fats stores are exhausted , body protein is again mobilized for energy by means of an increase in muscle protein degradation. This final increase in degradation of body protein cannot be sustained for long if feeding does not occur and death ensues. 2. Methionine and choline The most abundant phospholipids in eukaryotic cells are phosphatidylcholine and phosphatidylethanolamine. Both can be synthesized from phosphatidylserine or through alternative pathways that start with free choline or ethanolamine respectively. 3 methyl groups of choline are derived from amino acid methionine. Choline Phosphoatidycholine/ phosphatidyethanolamine

3. Glucagon and Lipid Glucagon promotes fatty acid oxidation resulting in energy production and ketone body synthesis. Fatty acid ___oxidation_______ ATP + ketone bodies Fat – Mineral inter-relation 1. Fats and Calcium An individual suffering from fat malabsorption shows decreased calcium absorption due to the formation of fatty acid soaps which are not absorbed and are excreted in faeces as ca soaps. Fat intake has a negative impact on ca balance only during steaorrhoea. Ca forms insoluble soaps with fatty acids in the gut.

2. Lipids and Phosphorus is bound with lipids to form phospholipids, like lecithin and cephlain, which are present in every cell membrane in the body These are the integral part of cell structure and also act as an intermediate in fat transport and absorption. 3. Iron and Fats Poor fat digestion leads to steaorrhoea which also leads to a decrease in iron absorption. 4. Fats and Sodium Bacterial action on CHO and fibers in large bowel generates short chain fatty acids: acetate, propionate and butyrate. These are widely absorbed and stimulates sodium absorption.

Fat – Vitamin inter-relation 1. Vitamin -c and Cholesterol a) Ascorbic acid participates in hydroxylation of certain steroid hormones synthesis in adrenal tissues. V- C con. decreases in periods of stress when adrenal cortical hormone activity is high. V-C when Adernal cortical hormone During periods of emotional , psychological stress , urinary excretion of V – C inreacses. b) The rate limiting step of bile acid synthesis in liver involves the Cholesterol 7 - alpha-hydroxylase The activity of this pathway is reduced in V – C deficient animals and is associated with elevated plasma cholesterol con. This leads to hypercholesterolemia.

3. Vitamin –A and Fat Retinoids and Cartenoids are incorporated into micelles along with other lipids for passive absorption into mucosal cells of small intestines. These then are incorporated chylomicrons for transport lymph and eventually blood stream which then finally pass to liver and tissues. The absorption of alpha , beta, gamma , carotene (provitamin A ) requires fat. In the absence of fat in diet , they are not absorbed. Rancid fats destroy the vitamin A and beta carotene present in the diet.

2. V- D and Cholesterol 2 sterols one in lipids of animals i. e. 7 -dehydrocholesterol and one in plants i. e ergosterol serve as precursor of VD And 7 -dehydrocholesterol under UV rays cholecalciferol(vitamin-D 3). Ergosterol Ergocalciferol(Vitamin D 2) Then these D 2 and D 3 require further metabolism to yield metabolically active form of 1, 25 dehydroxy vitamin D or cacitriol. Dietary vitamin D is incorporated into other lipids into micells and absorbed with lipids in intestine. Inside absorptive cells, vitamin is incorporated into chylomicrons , enters lymphatic system and subsequently enters plasma, where it delivered to cells.

4. Vitamin E and Fat Tocopherols act as antioxidant i. e. they can prevent the oxidation of various other oxidized substances such as fats and vitamin –A. It is located in the lipid portion of cell membranes it protects unsaturated phospholipids of the membrane from oxidative degradation from highly reactive oxygen species and other free radicals. Vitamin E perform this function through its ability to reduce such radicls into harmless metabolites by donating a hydrogen to them. This process is called free radicals scavenging


As a membrane free radicals scavenger, V – E is an important component of the cellular antioxidant defence system which involves other enzymes such as SOD(superoxide dismutases), glutathione peroxidases(GPxs), GR ( glutathione reductase, catalase and also non enzyme factors many of which depends on other essential nutrients. For eg – GSHxs, and TR depend on on slenium status etc. So, the antioxidant function of vitamin E can be affected by the levels of many other nutrients. Fatty acids with 2 or more double bonds i. e. polyunsaturated fatty acids are abundant in cell membrane and have important infulence on membrane fluidity and function. However , their double bonds make them susceptible to oxidation by free radicals. Fortunately , most V – E in body is found in cell membrane where it functions to protect polyunsaturated fatty acids from free radical attack. V- E stabilizes free radicals and prevent it from reacting with adjacent polyunsaturated

fatty acids. Also plasma lipoproteins, like cell membrane , contain an abundance of lipid including proportions of polyunsaturated fatty acids. They also contain fat soluble V- E which plays an essential role in protecting lipoproteins from oxidative damage. This is particularly important in low density lipoproteins (LDL) because lipid peroxides can oxidize apolipoprotein B resulting in formation of oxidatively modified LDL. apolipoprotein B LDL This oxidized LDL accumulates in walls of arteries at greater rate than normal LDL which is non oxidised , thus accelerating development of artherosclerotic plaques

�Morover, V – E is absorbed in manner similar to most other dietary lipids and requires fat digestion to be functioning normally. � The presence of fat in small intestine enhances V – E absorption because the products of triglyceride breakdown into gut promote the formation of mixed micells , the vehicle from which V – E is absorbed into the mucosal cell lining of the small intestine. �Lack of the bile acids or fat digestive enzymes damage to gastrointestinal wall or inability to synthesize chylomicrons which will decrease V – E absorption. � Disease in which V – E absorption is reduced includes pancreatic diseases and sometime other genetic inability to make chylomicrons.

5. Vitamin K and Fat Like other fat soluble vitamin absorption of V - K is also depends upon on minimum amount of dietary fat and on bile salts and pancreatic juices. The absorbed V – K is incorporated to chylomicrons in lymph and taken to liver , where are incorporated VLDL and subsequently delivered to to peripheral tissues by LDL. V- K VLDL Chylomicrons(lymph --- liver ) 6. Fat and Choline Tissues (LDL) Choline is a methyl rich essential component of animal tissues , where it is a structural unit of lecithin (i. e. phosphatidylcholine or phospholipids containing choline which is a part of bile where it emulsifies fats and is a part of lipoprotein also) and neurotransmitter acetylcholine. Thus choline is widely distributed in fats , existing predominantly in form of lecithin in eggs , liver, soyabeans , beef , milk and peanuts. Choline has several functions:

1. 2. 3. 4. 5. As phosphatidycholine, it is a structural element of membrane. A precursor to spingolipids ( lipids esters attached to spingosine base rather than glycerol and present in nervous system of animals and membrane of plants and lower eukaryotes such as yeast) A promoter to lipid transport As acetylcholine , it is a neurotransmitter. It functions as emulsifier in bile , thues helping with absorption of fat i. e. lipotropic factor and prevents accumulation of fat in liver i. e. it prevents fatty liver.