Chemical Reactions Review What is a compound Compound




- Slides: 10

Chemical Reactions

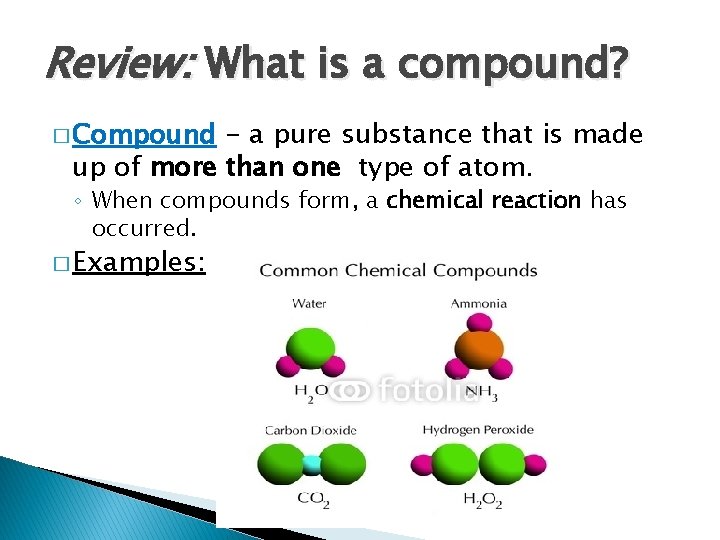
Review: What is a compound? � Compound - a pure substance that is made up of more than one type of atom. ◦ When compounds form, a chemical reaction has occurred. � Examples:

WHY do Elements form Compounds? �… To become more stable! � Example: � Na is flammable and Cl 2 is a toxic gas. � They combine to form Na. Cl (Sodium Chloride) � Na. Cl is a very stable compound that is neither flammable nor toxic

What if a Compound is unstable? � If compounds are unstable, then they will break down to form stable elements. � Example: � H 2 O 2 (Hydrogen Peroxide) will break down into Water (H 2 O) and release the extra Oxygen as a gas.

Video Clips � Why do elements form compounds? � http: //unctv. pbslearningmedia. org/resource/ nvhe. sci. chemistry. compounds/howelements-form-compounds/ � Why do elements react? � http: //unctv. pbslearningmedia. org/resource/ nvhe. sci. chemistry. reactive/what-makes-anelement-reactive/ � What makes radioactive elements unstable? � http: //unctv. pbslearningmedia. org/resource/ nvhe. sci. chemistry. fission/a-fission-chainreaction/

Review: Chemical Reaction � Chemical Reaction - 2 or more substances undergo a chemical change that results in the formation of a new substance. � Also known as a chemical change. � What are the indicators of a chemical reaction? � Evolution of Gas, Light, and/or Heat � Color Change � Formation of a Precipitate

Types of Chemical Reactions � Endothermic Reaction – A chemical reaction where the energy or heat is absorbed and the external temperature goes down. � Example: � The Baking Bread heat is absorbed in the bread during the chemical reaction, and the oven is cooler because of it. http: //www. youtube. com/watch? v=My. Azj. Sdc 3 Fc&fea ture=related

Types of Chemical Reactions � Exothermic Reaction - A chemical reaction where the energy or heat is released and the external temperature goes up. � Example: Fire ◦ Heat is being released into the environment and you can feel the temperature go up around the fire. http: //www. youtube. com/watch? v=YIf. V 2 Z-4 M 5 E

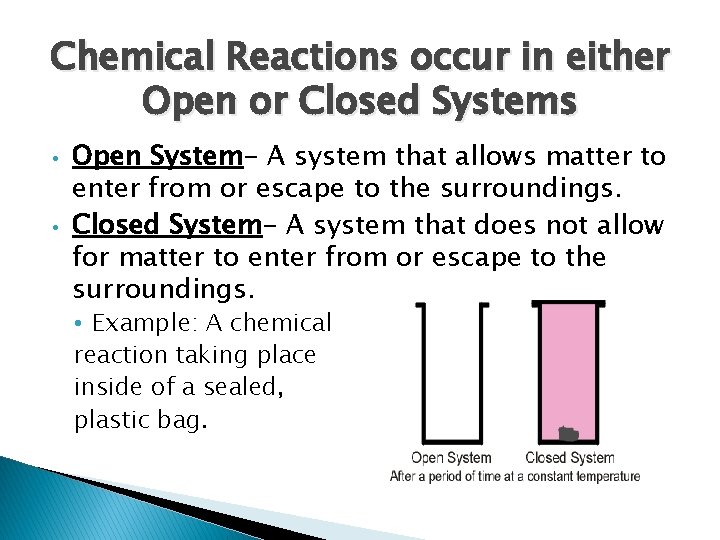
Chemical Reactions occur in either Open or Closed Systems • • Open System- A system that allows matter to enter from or escape to the surroundings. Closed System- A system that does not allow for matter to enter from or escape to the surroundings. • Example: A chemical reaction taking place inside of a sealed, plastic bag.

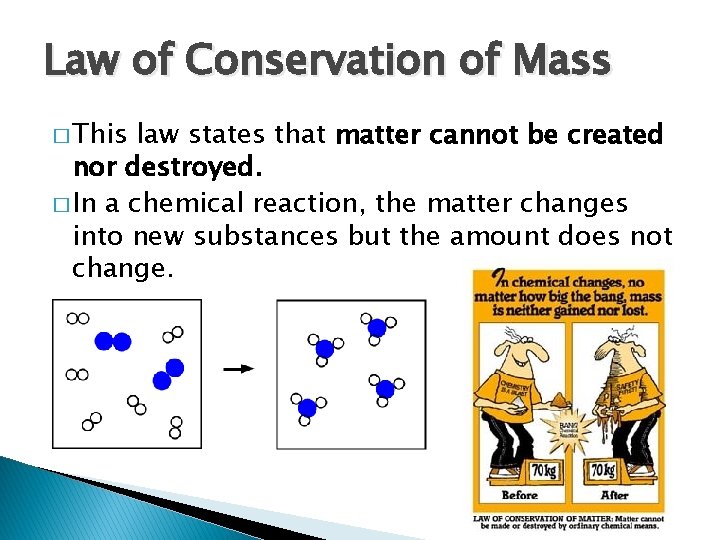
Law of Conservation of Mass � This law states that matter cannot be created nor destroyed. � In a chemical reaction, the matter changes into new substances but the amount does not change.
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Chapter 8 review describing chemical reactions
Chapter 8 review describing chemical reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Empirical formula pogil
Empirical formula pogil Chemical formulas and chemical compounds chapter 7 review
Chemical formulas and chemical compounds chapter 7 review Redox reaction example
Redox reaction example Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet



















