NUTRIENT CYCLES AKA The Water Cycle HYDROLOGICAL CYCLE
























- Slides: 30

NUTRIENT CYCLES

AKA The Water Cycle HYDROLOGICAL CYCLE

� Plants absorb water through their roots and expel water via their leaves. � Animals obtain water through their food or by drinking it. Animals give off water through breathing, perspiring and excretion. � Because there is only 1% of water on earth available to land organisms, it must be constantly recycled between the earth and the air.


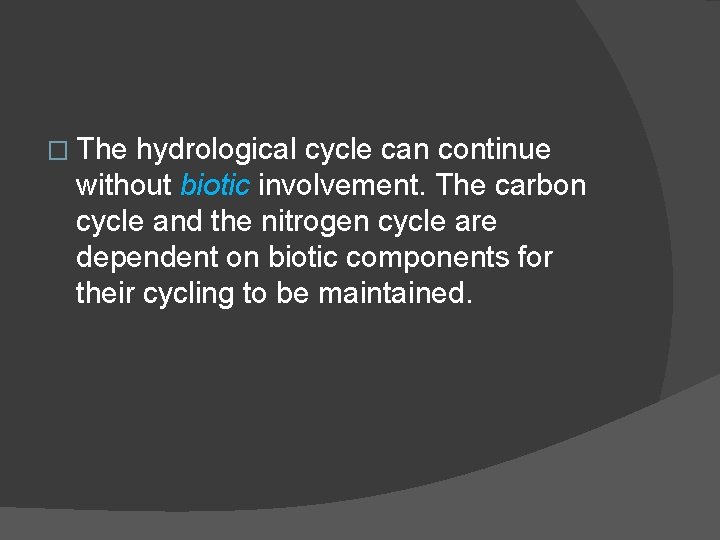
� The hydrological cycle can continue without biotic involvement. The carbon cycle and the nitrogen cycle are dependent on biotic components for their cycling to be maintained.

Questions: � � � 1. Calculate the percentages of water available as saltwater and in each other area of the biosphere. Reminder: 30 drops in 1 ml 2. Which of the four containers of freshwater represent the most freshwater on Earth? 3. Is this a source of freshwater commonly used by humans for drinking, watering lawns, etc. ? 4. Where is most of Earth's water found? 5. Can cities near oceans use the water from the oceans for households and industry? 6. Can salts be removed from water?

CARBON-OXYGEN CYCLE IN ECOSYSTEMS

� Carbon is a basic building block of all living materials. � Atmospheric carbon dioxide is one of the most important forms of carbon found in our biosphere. The carbon-oxygen cycle is very dependent on plants that photosynthesize solar radiation

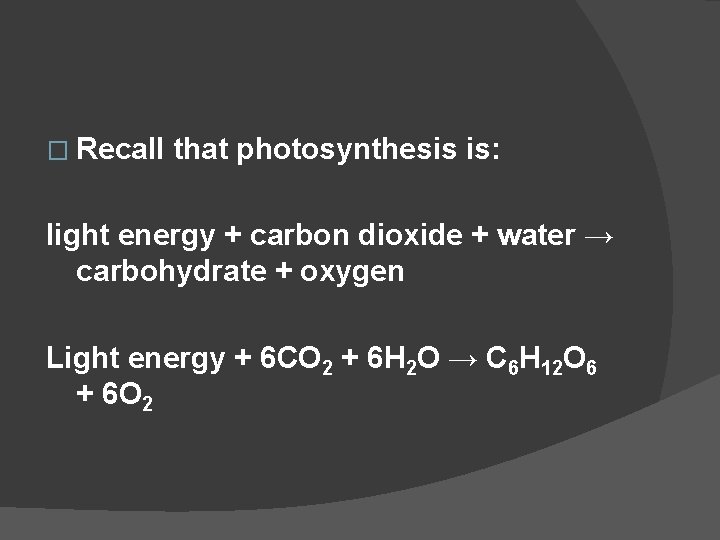
� Recall that photosynthesis is: light energy + carbon dioxide + water → carbohydrate + oxygen Light energy + 6 CO 2 + 6 H 2 O → C 6 H 12 O 6 + 6 O 2

� When plants or animals die, their remains are broken down and carbon dioxide is again released into the biosphere. Under suitable conditions, in swamp-like areas, peat can be compressed and slowly transformed into carbon-rich fossil fuels such as coal, natural gas and petroleum.


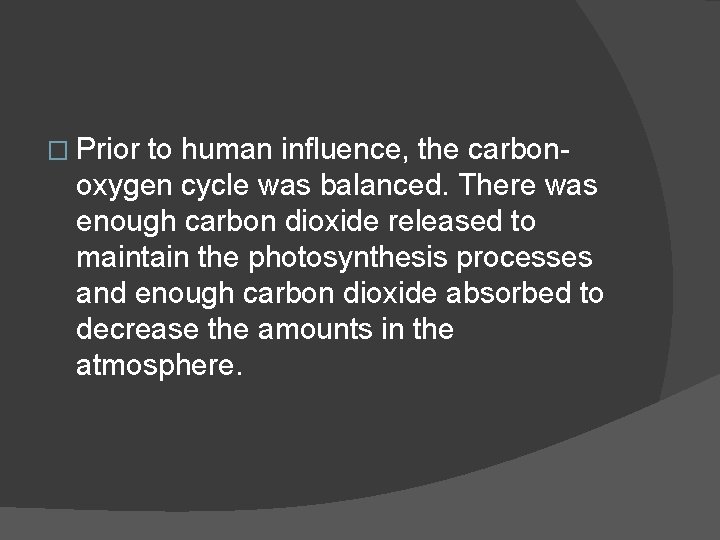
� Prior to human influence, the carbonoxygen cycle was balanced. There was enough carbon dioxide released to maintain the photosynthesis processes and enough carbon dioxide absorbed to decrease the amounts in the atmosphere.

However, human activities have abnormally increased the amount of carbon dioxide in the air. � Deforestation, the burning of fossil fuels, the use of aerosols have all had a huge impact on the stability of the carbon-oxygen cycle. The adverse effects of this instability is the increase in global warming and the destruction of the protective ozone layer in the stratosphere. �

NITROGEN CYCLE IN ECOSYSTEMS

� Nitrogen is an element that is necessary for the manufacture of proteins within all living organisms � The molecules that build DNA (deoxyribonucleic acid) also depend on the presence of nitrogen.

Unlike carbon and phosphorous, nitrogen is not found in large quantities in rocks and soil. � Even though nitrogen gas (N 2)is one of the most abundant gases in the atmosphere, it is in an unusable form for living organisms. � It must be converted into nitrates or nitrites before it can be used by plants or animals. Therefore, the nitrogen cycle involves the transformation and cycling of the various organic and inorganic forms of nitrogen within ecosystems. �


� The nitrogen gas from the atmosphere is 'fixed' by microorganisms in the soil into nitrate form that can then be taken in by the roots of plants and enter into the food chain as proteins

The most common microorganism that 'fixes' the nitrogen are bacteria that live in a symbiotic relationship in nodules on the roots of some legume plants. � Other microorganisms: � �are species of lichens which are species of fungi and algae that live in a mutualistic relationship. �There are some free-living bacteria such as the cyanobacteria.

�A small amount of nitrogen gas is converted into nitrates and nitrites in the atmosphere through electric lightning storms that touch the earth. The nitrogen gas is mixed with oxygen gas in the atmosphere under the pressure and heat of the storm.

� When organisms die, the nitrogen within their bodies must be converted to an inorganic form to complete the cycle. A process called ammonification is the converting of organic nitrogen to ammonium (NH 4+) by the addition of hydrogen ions

This process is completed by microorganisms in the soil. � Some plant species can use the ammonium for their source of nitrogen, but most plants require further processing of the nitrogen. � Microorganisms convert the ammonium into nitrites and then into nitrates. The changing of ammonium into nitrites and nitrates is called nitrification. �

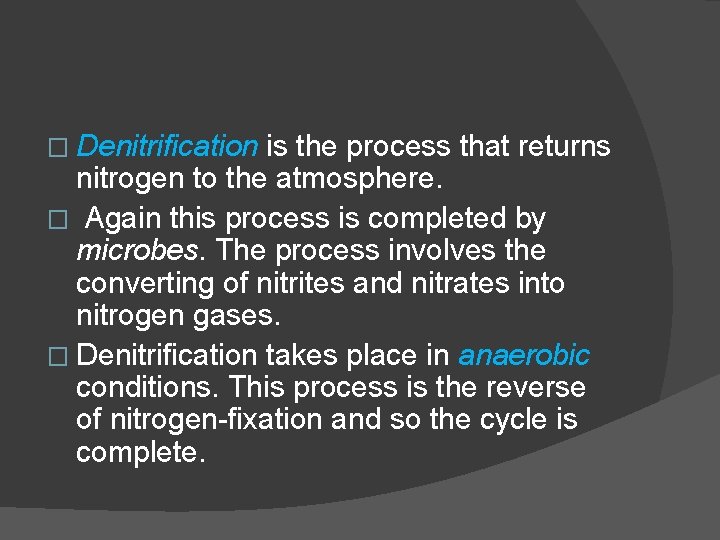
� Denitrification is the process that returns nitrogen to the atmosphere. � Again this process is completed by microbes. The process involves the converting of nitrites and nitrates into nitrogen gases. � Denitrification takes place in anaerobic conditions. This process is the reverse of nitrogen-fixation and so the cycle is complete.


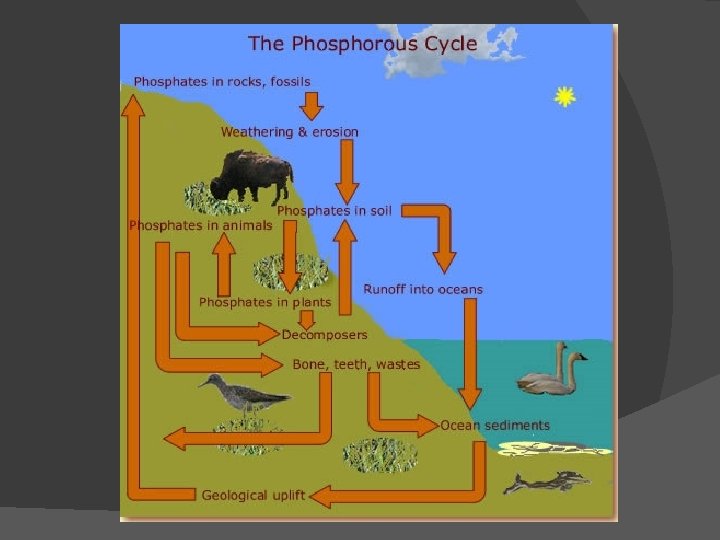
PHOSPHOROUS CYCLE IN ECOSYSTEMS

� Phosphorous is an important component in many biochemicals including the molecules that are responsible for our genetic code (DNA - deoxyribonucleic acid), in the formation of strong bones and in the membranes of cells. � The body's requirement for phosphorous, however, is a great deal smaller than its need for carbon or nitrogen.

� The majority of phosphorous on Earth is found in rocks and soil. As rock erodes it releases phosphorous into the soil in the form of phosphate ions (PO 4 -3). � Phosphorous is water soluble so it can be absorbed through the roots of plants and then passed on to herbivore organisms and along the food chain.

� Some phosphorous runs-off into lakes, streams, rivers and eventually settles in the ocean bottom. It remains there unless there is some force that physically returns it to the terrestrial areas.

� These forces would vary from substantial tectonic plate movement resulting in mountain formation to migrating fish such as salmon that return inland to spawn and die. Decomposers than break down the dead and decaying material and return phosphorous to the soil. Fish-eating birds return phosphorous to the land in their excrement
